Mammary gland cells
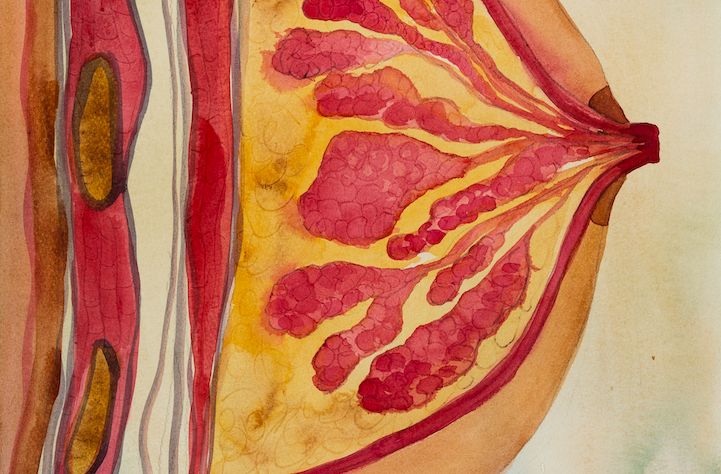
Biorelevant culture of human mammary cells on Biolaminin substrates
Laminins have both overlapping and unique functions in the mammary gland tissue
Mammary ducts consist of an epithelial layer with a basal connection to a single layer of myoepithelial cells. The duct is encircled by a basement membrane sheet that is intimately connected with the myoepithelial cells (Fata, 2003). The mammary basement membrane mainly contains laminin isoforms 111, 332, 511, 521 and collagen IV. Changes in the basement membrane laminin composition influence stem cell maintenance and mammary development, but also plays a role during aging and in aging-related diseases, such as cancer (Hu, 2017; Fata 2003). The extracellular matrix not only helps to support mammary basic structure but also serves as a communicating bridge between mammary epithelia and their local and global environment throughout development. The major integrin receptors in the mammary gland are α3β1, α6β1 and α6β4 and the interaction between mammary epithelial cells and their extracellular matrix is crucial in the development and function of the tissue, affecting cell survival, polarity, proliferation, differentiation, adhesion, and migration (Hu, 2017; Muschler, 2010; Fata, 2003).
Laminin 111 is important for the initiation of mammary gland development, for ductal elongation and end bud persistence
End buds are surrounded by a basement membrane that is rich in laminin 111 and collagen IV. Blocking laminin 111 or β1 integrins specifically and dramatically reduced both the number of end buds per gland and the extent of the mammary ductal network (Klinowska, 1999). Laminin 111 and laminin 332 are suggested to be involved in the initiation of mammary gland development, where laminin 111 has been shown to be critical for ductal elongation and end bud persistence (Chia, 2007; Hu, 2017; Muschler, 2010). Laminin 111 is also important for mammary epithelial cells to form polarized acinar structures, express b-Casein and permit prolactin receptor signaling (Hu, 2017; Muschler, 2010).
Laminin 511 and laminin 521 control submandibular gland epithelial morphogenesis and is required for correct epithelial cell organization and lumen formation
Laminin 511 is abundant in the vasculature of normal human mammary glands (Chia, 2007). Published results show that laminin 511 is not essential for gland initiation, but plays an important role in the initial cleft formation and epithelial morphogenesis. Laminin 511 is also necessary for sublingual gland formation and later in development, is required for epithelial cell organization and lumen formation (Rebustini, 2007; Hu, 2017; Muschler, 2010). In submandibular glands, laminin 511 and laminin 111 are present in epithelial clefts but during epithelial morphogenesis as branching begins, laminin 511 expression increases while laminin 111 decreases. The data suggest that submandibular gland epithelial morphogenesis is controlled by α5-chain laminins (laminin 511 and 521), regulated via β1 integrin signaling. Laminin 511 also regulates FGFR expression, independent of laminin 111, which reciprocally regulates the expression of the laminin α5 subunit (Rebustini et al., 2007).
Laminin 511 and 521 promotes breast cancer cell migration and invasion
The laminin 511 and 521 isoforms are expressed in high levels in breast tumors (Chia, 2007) and are suggested to play an autocrine role in mammary cancer progression (Chia, 2007). Laminin 111 and laminin 332 are both down-regulated in most advanced breast tumors, suggesting a tumor-suppressive role (Muschler, 2010). On the contrary, laminin 511 acts as a functional substrate, regulating breast cancer metastasis. Laminin 511 is a potent adhesive substrate for both murine and human breast carcinoma cells and promotes strong haptotactic responses in metastatic lines, largely mediated by the α3 integrin (Chia, 2007; Kusuma, 2011). Chia et al. propose that laminin 511 contributes during the process of metastasis via the attachment of breast tumor cells to blood vessel walls. These results are in accordance with a publication by Chang et al. where the authors show that breast cancer stem cells produce alpha-5 laminin matrix that promotes self-renewal and tumor initiation via α6β1 integrin interaction. α5 chain laminins also activate the Hippo transducer TAZ which in turn regulates the transcription of the α5 laminin subunit, establishing a positive feedback loop that contributes to stemness in breast cancer (Chang, 2014).
Protocol:
Succeed with your application
-
Evidence for a Role of Tumor-Derived Laminin-511 in the Metastatic Progression of Breast Cancer
Chia J., Kusuma N., Anderson R., Parker B., Bidwell b., Zamurs L., Nice E., Pouliot N. The American Journal of Pathology, 2007
Read more -
Instructions 001: Coating with Biolaminin substrates
Protocol and concentration calculations for coating cultureware with Biolaminin
Open pdf
Biolaminin Key Advantages
The interaction between mammary epithelial cells and their extracellular matrix is crucial in the development and function of the tissue. Laminin 111 is important for the initiation of mammary gland development, critical for ductal elongation and end bud persistence. Laminin 511 plays an important role in the initial cleft formation, is necessary for sublingual gland formation, and is required for epithelial cell organization and lumen formation. Laminin 511 is expressed in high levels in breast tumors and promotes breast cancer cell migration and invasion.
Specific laminin isoforms are present in different tissue microenvironments and are essential for cell survival, proliferation, and differentiation. Biolaminin products allow you to imitate the natural cell-matrix interactions in vitro.
Our products have consistent composition and quality. This enables minimized variability between experiments.
All our matrices are chemically defined and animal origin-free, which makes them ideal substrates for each level of the scientific process – from basic research to clinical applications.
Numerous scientists have found our products and finally succeeded in their specific stem cell application. The power of full-length laminins incorporated into various cell systems is well documented in scientific articles and clinical trials.
Recommended products
-

Biolaminin 511 LN (LN511)
Full-length human recombinant laminin-511
Biolaminin 511 is the natural laminin for mouse embryonic stem cells, supporting sustained pluripotency without the need for LIF. It also efficiently promotes the culture of many tissue-specific human cell types.View product -

Biolaminin 521 LN (LN521)
Full-length human recombinant laminin-521
Biolaminin 521 LN is a full-length laminin-521 substrate—the natural laminin for pluripotent stem cells, reliably facilitating ESC and iPSC self-renewal in a chemically defined, xeno-free stem cell culture system. It also uniquely promotes the growth and identity of various tissue-specific human cell types.View product -

Biolaminin 111 LN (LN111)
Full-length human recombinant laminin-111
Biolaminin 111 is a full-length laminin-111 protein—an essential extracellular matrix component for many cell types in vivo. It has proven particularly effective in supporting the differentiation of functional hepatic and neural cells in a chemically defined and xeno-free culture.View product -

Biolaminin 121 LN (LN121)
Full-length human recombinant laminin-121
Biolaminin 121 is a full-length human laminin-121 protein that can be used as a general attachment protein for many cell types in vitro.View product
Talk to our team for customized support
We are here to help you in your journey.