Publications
Here is a selection of publications where different laminin isoforms were used to create more authentic cell culture systems.
-
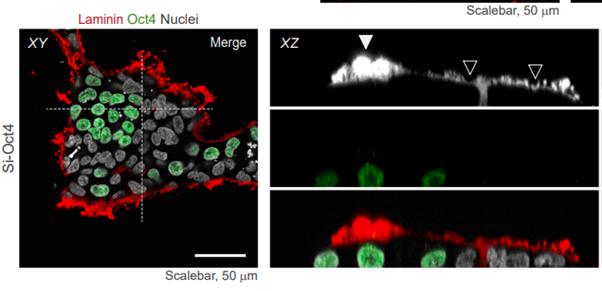
Recent studies show why stem cells need full-length laminin substrate
Recent articles highlighting human embryogenesis and intact laminin functionality show why stem cells prefer full-length laminin-521 substrate.
-
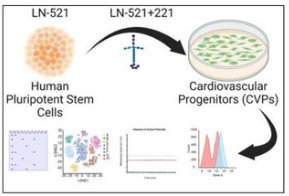
Pluripotent stem cell-derived committed cardiac progenitors remuscularize damaged ischemic hearts and improve their function in pigs. npj Regenerative Medicine, 2023
Lynn Yap, Li Yen Chong, Clarissa Tan, Swarnaseetha Adusumalli, Millie Seow, Jing Guo, Zuhua Cai, Sze Jie Loo, Eric Lim, Ru San Tan, Elina Grishina, Poh Loong Soong, Narayan Lath, Lei Ye, Enrico Petretto & Karl Tryggvason.
Dr. Lynn Yap and colleagues present pre-clinical evidence of their promising myocardial infarction treatment using stem cell-derived committed cardiac progenitors (CCPs). The CCPs were differentiated from human embryonic stem cells on Biolaminin 521 and Biolaminin 221 matrices, maturing and improving heart function in pigs post-transplantation. The study highlights reduced ventricular arrhythmia risk, enhanced left ventricular ejection fraction, improved ventricular wall thickness, and reduced infarction size, with all pigs surviving without adverse effects, marking a notable advancement in this field.
-
Observing Dynamic Conformational Changes within the Coiled-Coil Domain of Different Laminin Isoforms Using High-Speed Atomic Force Microscopy
Akter L, Flechsig H, Marchesi A, and Franz CM. Int. J. Mol. Sci. 2024
This study by Akter and colleagues provides a visual insight into the significance of using whole, full-length laminin to mimic biological conditions in cell culture settings. Even though advanced microscopy has enabled the imaging of cross-shaped laminin proteins before, these methods could only provide snapshots of individual conformational states or only low-resolution dynamic images without submolecular details. Here, an optimized method was developed for studying the dynamic motion of full-length laminin protein under physiological conditions using an optimized high-speed atomic force microscopy method. The cell adhesion domain of the examined laminin protein isoforms (Biolaminin LN111 and LN332) displayed structural dynamism, alternating between open and compact conformations. This suggests that the cell-binding interface could be more dynamic than previously thought, potentially influencing adhesion receptor binding. As a multi-adapter protein, laminin has roles in binding to both cells and the extracellular matrix network. The configuration of its domains along the protein influences the presentation of the cell binding domain to cells and modulates interactions with the surrounding extracellular matrix network. Video S4 in the supplemental data shows the dynamic movements of laminin-111.
-
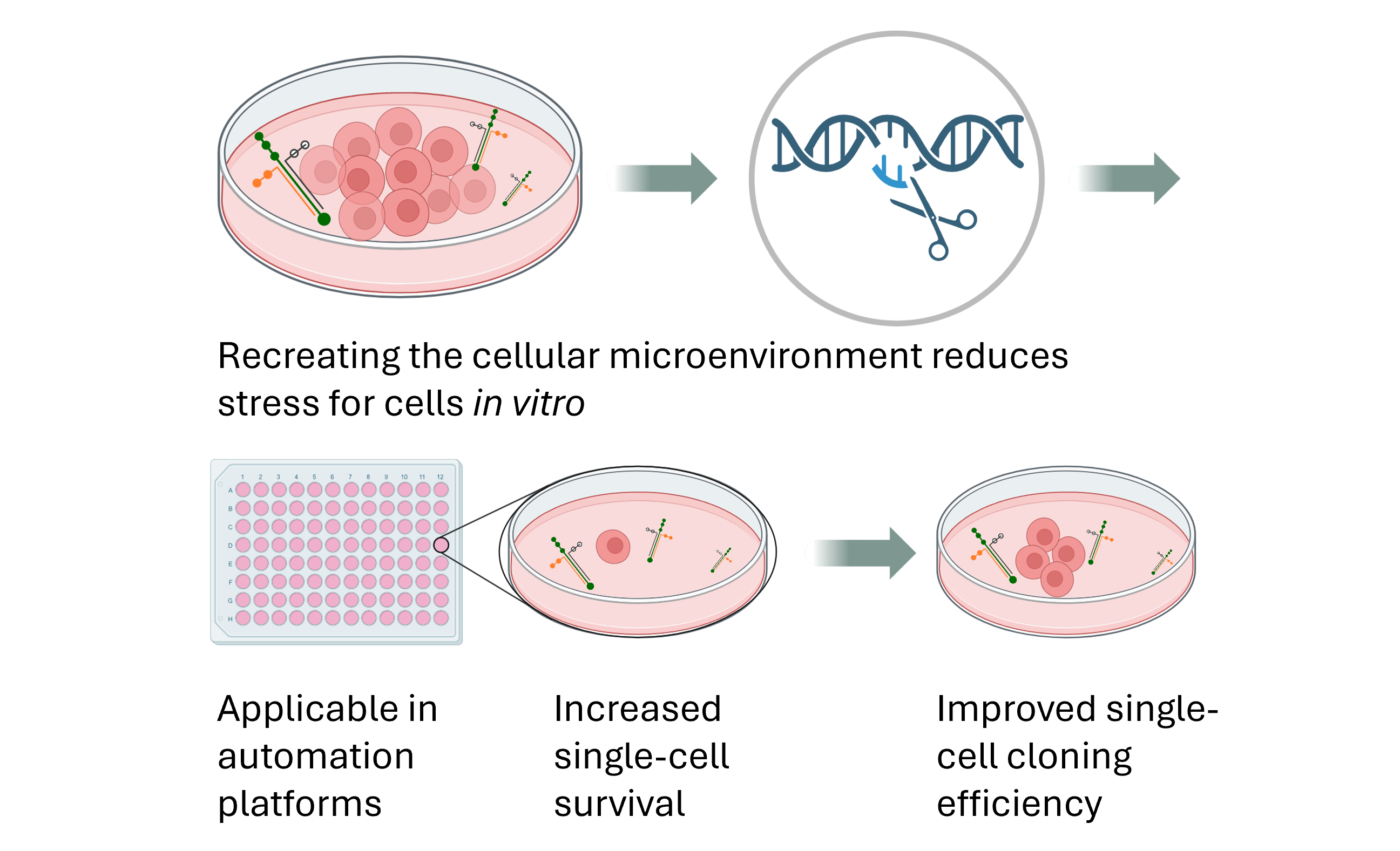
Integration of xeno-free single-cell cloning in CRISPR-mediated DNA editing of human iPSCs improves homogeneity and methodological efficiency of cellular disease modeling
Atefeh Namipashaki, Kealan Pugsley, Xiaodong Liu, Kirra Abrehart, Sue Mei Lim, Guizhi Sun, Marco J. Herold, Jose M. Polo, Mark A. Bellgrove, and Ziarih Hawi. Stem Cell Reports, 2023
This article presents an improved method for generating genetically homogeneous induced pluripotent stem cell (iPSC) clones following CRISPR-Cas9 genome editing. The greatest single-cell clone survival rate, 70 %, was noted when the human full-length laminin-521 (Biolaminin 521, LN521) surface matrix was applied, with a substantial increase from the 36% survival rate observed when vitronectin was utilized. The method employs ribonucleoproteins and lipid transfection. Validation of the approach across three iPSC lines consistently showed high editing efficiencies. The resulting cloned iPSC lines retained pluripotency, differentiation potential, and genomic stability.
-
Leveraging interacting signaling pathways to robustly improve the quality and yield of human pluripotent stem cell-derived hepatoblasts and hepatocytes
Claudia Raggi, Marie-Agnès M’Callum, Quang Toan Pham, Perrine Gaub, Silvia Selleri, Nissan Vida Baratang, Chenicka Lyn Mangahas, Gaël Cagnone, Bruno Reversade, Jean-Sébastien Joyal, Massimiliano Paganelli. Stem Cell Reports, 2022
This study describes a robust and scalable protocol for pluripotent stem cell (PSC) differentiation into high-quality bipotent hepatoblasts and hepatocyte-like cells (HLCs), with Biolaminin 521 (laminin-521) as the cell culture matrix. The protocol resulted in cells that had a better resemblance to primary human hepatocytes than protocols described previously, showing a good potential to use them as an alternative to primary cells for in vitro modeling. PSC-derived hepatoblasts were able to mature into hepatocytes and bile ducts within syngeneic liver organoids.
-
Protocol for the derivation, culturing, and differentiation of human iPS-cell-derived neuroepithelial stem cells to study neural differentiation in vitro
Javier Calvo-Garrido, Dania Winn, Camilla Maffezzini, Anna Wedell, Christoph Freyer, Anna Falk, Anna Wredenberg. STAR Protocols, 2021
This protocol describes the derivation of neuroepithelial stem (NES) cells from human induced pluripotent stem cells. NES cells can be further differentiated into neurons and glia. The PSC culture and NES differentiation were done on plates coated with Biolaminin 521 (laminin-521). To avoid clonal selection of isolated NES cells, it is recommended to follow the culture conditions described. The protocol is expected to result in highly proliferate NES cells providing a good source of cells of a neuronal cell lineage. Glial cells are formed after approximately 45 days of differentiation.
-
CRISPRi-mediated transcriptional silencing in iPSCs for the study of human brain development
Pia Annette Johansson, Anita Adami, Johan Jakobsson. STAR Protocols, 2022
This protocol describes using CRISPRi-mediated transcriptional silencing in human induced pluripotent stem cells. In addition, it contains an efficient protocol for neural progenitor differentiation. The method is directly applicable to loss-of-function studies in brain development research. Biolaminin 521 (laminin-521) is applied as the cell culture matrix for PSCs and Biolaminin 111 (laminin-111) for hiPSC differentiation into forebrain neural progenitor cells. The protocol is expected to achieve high transduction and silencing efficiency. After two weeks of differentiation, the authors detected no reduction in the percentage of positive cells, and the cerebral organoids had stable transcriptional silencing even after 4 months.
-
Generation of a CHIP isogenic human iPSC-derived cortical neuron model for functional proteomics
Catarina Dias, Erisa Nita. Jakub Faktor, Lenka Hernychova, Tilo Kunath, Kathryn L. Ball. STAR Protocols, 2022
This protocol describes the production of gene-edited cells using CRISPR-Cas9 and a patient-derived induced pluripotent stem cell (iPSC) line. Biolaminin 521 was used as the matrix to improve single cell survival and pluripotency. The resulting panel of iPSC lines was differentiated into cortical neurons with the Biolaminin 111 culture matrix. The overall aim was to identify protein and pathway targets for the neuroprotective E3-ubiquitin ligase CHIP, which is important in healthy brain aging. The protocol can be adapted to other proteins and pluripotent stem cell lines.
-
Methods for Automated Single Cell Isolation and Sub-Cloning of Human Pluripotent Stem Cells
Valeria Fernandez Vallone, Narasimha Swamy Telugu, Iris Fischer,Duncan Miller, Sandra Schommer, Sebastian Diecke, Harald Stachelscheid. Current Protocols in Stem Cell Biology, 2020
This publication describes automated workflows to facilitate high-throughput hPSC clonal selection and expansion, which is required for example after genome editing. Three different automated single cell dispensing devices were applied with Biolaminin 521 coating of plates and multiwells. Laminin-521 increases single cell cloning efficiency compared to other commonly used matrices. Single cell−derived hPSC sub-clones from each system maintained pluripotency and genetic stability.
-
Astrocytic laminin-211 drives disseminated breast tumor cell dormancy in brain
Jinxiang Dai, Patrick J. Cimino, Kenneth H. Gouin III, Candice A. Grzelak, Alexander Barrett, Andrea R. Lim, Annalyssa Long, Stephanie Weaver, Lindsey T. Saldin, Aiyedun Uzamere, Vera Schulte, Nigel Clegg, Laura Pisarsky, David Lyden, Mina J. Bissell, Simon Knott, Alana L. Welm, Jason H. Bielas, Kirk C. Hansen, Frank Winkler, Eric C. Holland and Cyrus M. Ghajar. Nature Cancer, 2022
This study shows that astrocyte-deposited laminin-211 drives disseminated tumor cell (DTC) quiescence in brain metastases by inducing the dystroglycan receptor. Dormancy has a key role in the metastasis of breast cancer cells to the brain. The authors compared the laminin 211 isoform with laminin 411, 511, and 111, and only 211 significantly and substantially reduced breast cancer cell outgrowth in addback experiments.