How do laminin proteins interact with each other and attach to the cell culture well surface?
In vivo, the composition of the basement membrane is highly selective to specific cell surfaces, with laminins playing a key role in its assembly. Laminin molecules self-assemble through a thermodynamically unfavorable nucleation binding, followed by calcium-dependent polymerization of the LN domains in the short arms of the α, β, and γ chains (Yurchenco et al., 1985; Carafoli et al., 2012; Yurchenco & Cheng, 1993; Purvis & Hohenester, 2012). This process results in the formation of a sheet-like laminin network that binds to other proteins in the basement membrane. Laminin interacts with nidogen via LE motifs on the γ1 and γ3 chains (Gersdorff et al., 2005; Takagi et al., 2003; Stetefeld et al., 1996), and the Lβ domain of the β chains binds to agrin (Domogatskaya et al., 2012). Additionally, laminin is linked to collagen IV through nidogen and heparin interactions, forming a covalently stabilized network (Hohenester & Yurchenco, 2013).
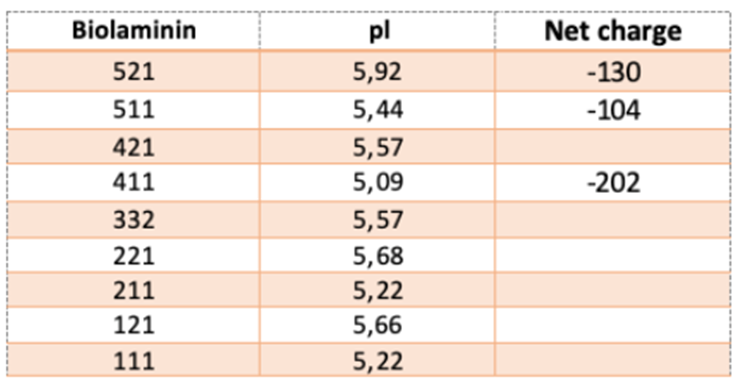
Laminins are large proteins with both hydrophobic and hydrophilic regions, enabling them to interact with artificial surfaces. The LG modules contain the highest density of anionic (negative) charges, which may be critical for laminin binding and self-assembly. Tissue-culture treatment involves modifying polystyrene surfaces to become hydrophilic, typically by increasing negative charge chemically. This increased negative charge is essential for cell attachment and may explain why cells cultured in non-treated plasticware tend to clump, as they are unable to adequately attach to the surface.
The net charge and isoelectric pH (pI) of Biolaminin proteins are displayed in the table.