How do laminin proteins interact with each other and how do they attach to the cell culture well surface?
In vivo, basement membrane composition is highly cell-surface selective and for proper assembly, laminins are the key proteins. Laminin molecules self-assemble via a thermodynamically unfavorable nucleation binding followed by a calcium-dependent polymerization of the LN domains in the short-arms of the α, β, and γ chains (Yurchenco et al., 1985; Carafoli et al., 2012; Yurchenco & Cheng, 1993; Purvis & Hohenester, 2012). The sheet-like laminin network binds to other proteins in the basement membrane. Laminin interacts with nidogen via LE motifs of the γ1 and γ3 chains (Gersdorff et al., 2005; Takagi et al., 2003; Stetefeld et al., 1996), and the Lβ domain of the β chains binds to agrin (Domogatskaya et al., 2012). Laminin is linked to collagen IV through nidogen and heparin interactions, forming a covalently stabilized network (Hohenester & Yurchenco, 2013).
Laminins are large proteins with both hydrophobic and hydrophilic sites that allow them to interact with artificial surfaces. The highest density of anionic (negative) charges in laminins is to be found in the LG modules. It has been suggested that the density of negative charges on the surface could be critical for laminin-binding and self-assembly. Tissue-culture treatment is a process by which polystyrene surfaces are made to become hydrophilic, usually by increasing negative charge through a chemical means. This increased negative charge is important for cell attachment and may explain why cells cultured in non-treated plastic ware clump up since they are not attaching adequately to the surface.
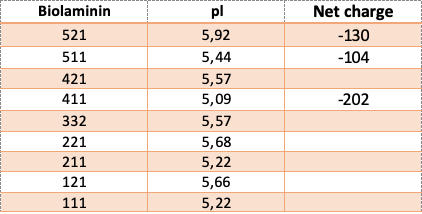
The net charge and isoelectric pH (pI) of the Biolaminin proteins are displayed in the table.