Scientific Spotlight – 2024-10
Recent studies show why stem cells need full-length laminin
Learn from recent scientific publications how full-length laminin-521 (LN521) critically supports stem cell survival and development.
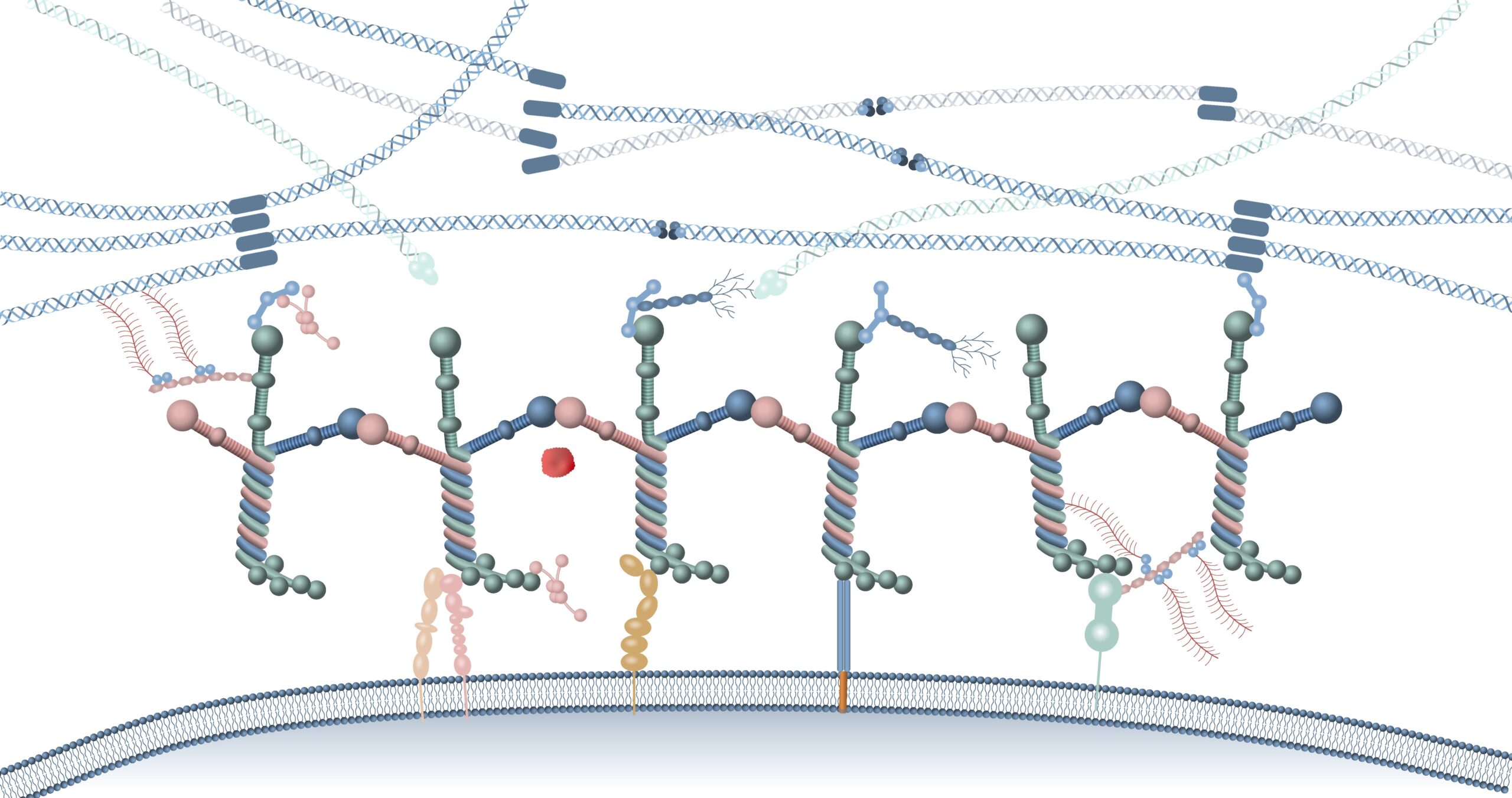
Laminins are essential extracellular matrix (ECM) proteins that support cell identity and growth in vivo from the earliest stages of development. In stem cell culture, recombinant full-length laminin-521 mimics the natural stem cell niche, promoting pluripotency, survival, and efficient proliferation. Recent articles highlight the significance of full-length laminins for cell functionality.
The first protein in the pluripotent stem cell niche: laminin-521
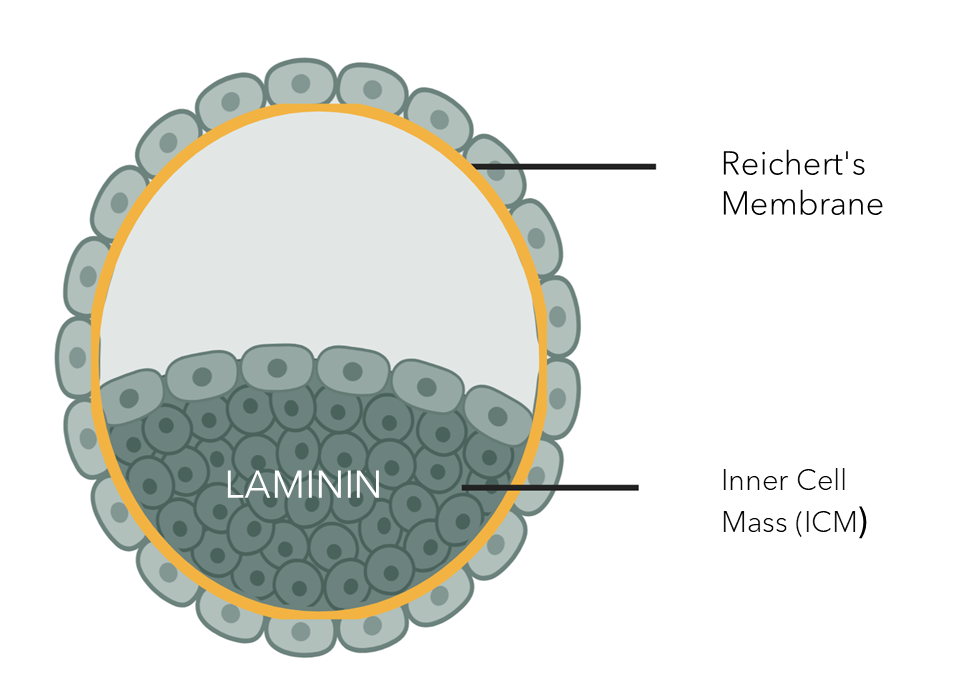
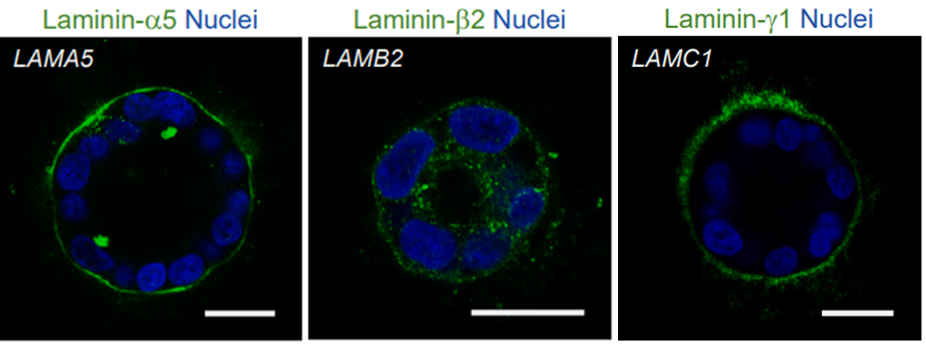
A recent publication by Rosner and Hengstschläger (2024) in Developmental Cell identifies laminin-521 (LN521) as a critical component of the basement membrane (BM) during human embryogenesis. The basement membrane is a specialized layer of the extracellular matrix that provides structural support, regulates cell behavior, and is essential for cell survival, differentiation, and tissue organization.
Key findings include:
✔ LN521 is the first and foundational component of the embryonic BM, supporting stem cells from the earliest stages.
✔ Once produced, LN521 binds directly to cell surfaces and self-assembles into an independent network.
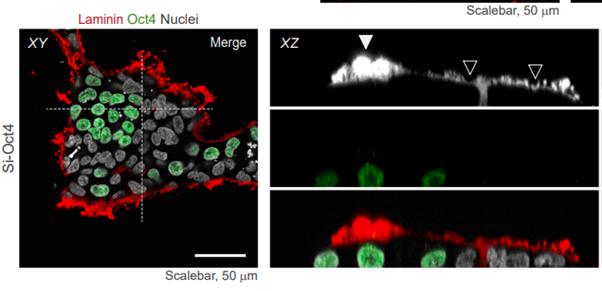
✔ LN521 regulates cell differentiation and tissue development from the early stages of embryogenesis.
✔ Oct4 regulates laminin assembly via Rac1 and Akt signaling pathways.
Proper formation and maintenance of these laminin-containing BMs are crucial for tissue formation during early human development.
What about other laminin isoforms, such as laminin-511?
The article notes an interspecies difference between the human and mouse pluripotent stem cell (PSC) niche: laminin-511 (LN511, which contains the beta1 chain) is present in the mouse embryo, whereas laminin-521 (beta2) is found in humans. LN521 is recognized as the biologically relevant isoform for human PSCs. Additionally, the study reports that laminin-111 (LN111) is restricted to the outer layer of the human embryo (Bruchert’s membrane) and therefore it holds no significance for human PSCs.
Full-length laminins: essential for proper cell-ECM interactions
Full-length laminin means full functionality.
A recent comprehensive review by renowned laminin experts Yurchenko and Kulczyk (2024) in Journal of Biological Chemistry explains how full-length laminins self-assemble and interact with extracellular matrix (ECM) components and various receptors on the cell membrane. These interactions are critical for maintaining tissue integrity and supporting cellular functions.
Minor changes in laminin composition can cause diseases such as muscular dystrophy and Pierson syndrome, underscoring the need for intact, full-length laminin to support healthy tissues.
Key aspects of full-length laminins include:
✔ Functional domains: Full-length laminins retain critical domains for versatile interactions with other ECM proteins, which are missing in truncated forms.
✔ Polymerization and assembly: Full-length laminins are necessary for proper basement membrane formation, establishing stable networks that support cell function.
✔ Comprehensive interactions: Only full-length laminins bind all relevant receptors (such as dystroglycans and syndecans), other ECM components (like perlecan and nidogen), and growth factors (such as VEGF).
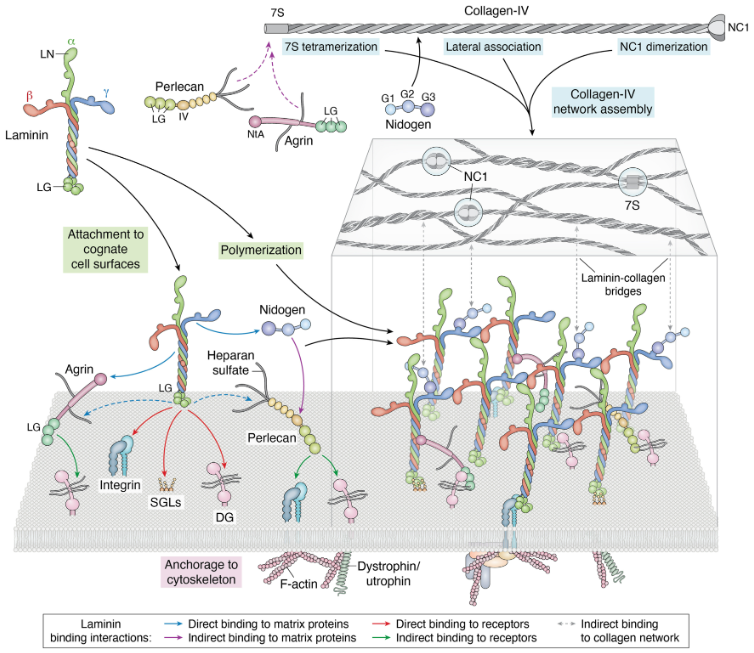
These findings show that only full-length laminin-521 can accurately replicate the natural stem cell environment in vitro.
Human recombinant full-length laminin: the biologically relevant cell culture matrix
Laminins are large heterotrimeric proteins that are almost impossible to extract in the full-length format from tissues. Using fragmented or truncated laminins in cell culture can compromise the environment required for proper cell signaling and function, as these forms lack key domains necessary for complete interactions. Even though producing the large laminin proteins recombinantly is challenging, it is possible with the correct advanced methodology.
Unlike fragments, the full-length laminins provide:
✔ Complete signaling. Full-length laminins retain all binding sites, ensuring proper signaling for cell identity, differentiation, and growth.
✔Stable environment. Full-length laminins create robust networks that provide stability for sensitive cells like stem cells and allow migration.
✔ In vivo mimicry. Full-length laminin-521 is the foundational stem cell niche protein able to replicate natural ECM interactions.
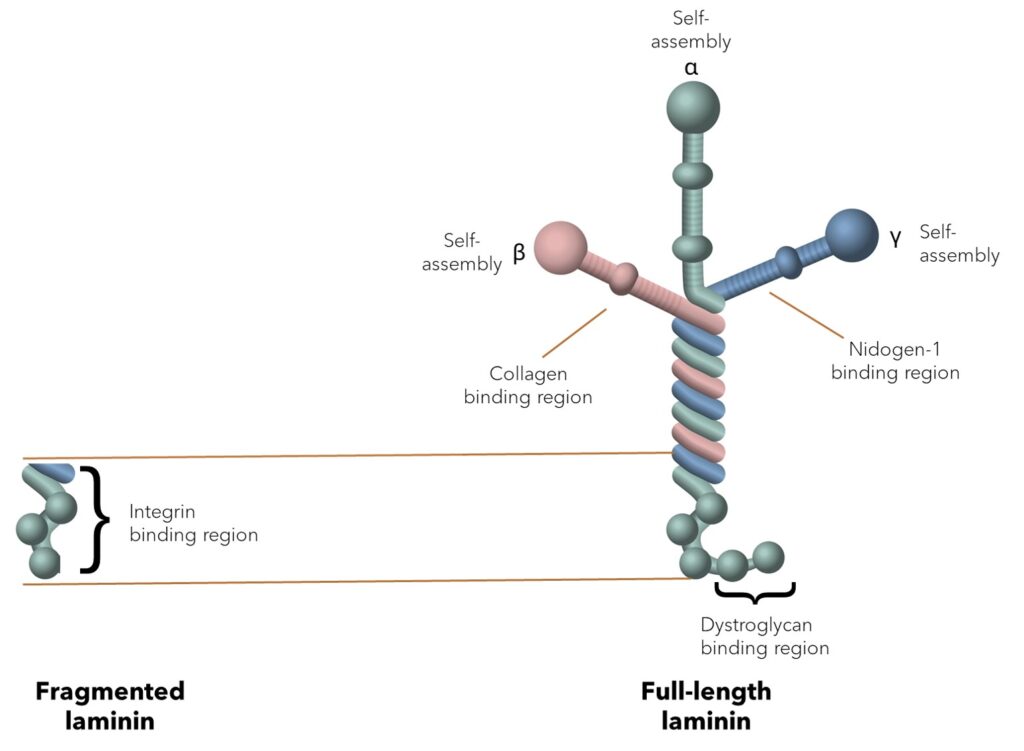
The structural features of full-length LN521 directly translate into key benefits for PSCs in culture:
✔ Maintenance of pluripotency without spontaneous differentiation
✔ High cell survival during single-cell passaging and cloning, even without ROCKi
✔ Maximized stability and optimal use of the culture area, supporting cell migration
✔ Enhanced proliferation with up to 10x more cells in 10 days
Laminin interactions revealed by advanced methodologies
Recent advances in high-resolution techniques, such as cryo-electron microscopy and atomic force microscopy (AFM), combined with AlphaFold2 modeling and biochemical studies, have revealed how laminin domains interact and contribute to cell adhesion and signaling.
In one of the recent studies, Akter and colleagues used high-speed AFM to capture the dynamic movements of laminin isoforms in real-time (Akter et al. 2024, Int J Mol Sci). The findings suggest that the cell-binding interface could be more dynamic than previously thought, potentially influencing adhesion receptor binding.
Want to go further?



Read more in these publications
Oct4 controls basement membrane development during human embryogenesis.
Rosner M. & Hengstschläger M. 2024 Developmental Cell
Polymerizing laminins in development, health, and disease.
Yurchenco PD, Kulczyk AW. 2024 JBC Reviews
Observing dynamic conformational changes of laminin isoforms using high-speed atomic force microscopy.
Akter L, Flechsig H, Marchesi A, Franz CM. 2024 International Journal of Molecular Sciences

Talk to our team for customized support
We are here to help you in your journey.