Scientific Spotlight
Instant single-cell recovery after genome editing
Full-length laminin-521 matrix significantly enhances single-cell survival of stem cells, boosting cloning efficiency for both basic research and clinical applications.
- Namipashaki A, Pugsley K, Liu X, Abrehart K, Lim SM, Sun G, Herold MJ, Polo JM, Bellgrove MA, and Hawi Z. Stem Cell Reports, 2023.
Integration of xeno-free single-cell cloning in CRISPR-mediated DNA editing of human iPSCs improves homogeneity and methodological efficiency of cellular disease modeling.
Xeno-free single-cell cloning of human iPSCs improves the homogeneity and efficiency of cell modeling
This article presents an improved method for generating genetically homogeneous induced pluripotent stem cell (iPSC) clones following CRISPR-Cas9 genome editing. The method employs ribonucleoproteins and lipid transfection. Validation of the approach across three iPSC lines consistently showed high editing efficiencies. The resulting cloned iPSC lines retained pluripotency, differentiation potential, and genomic stability.
Full-length laminin is crucial for cells in vivo and in vitro
Over a decade ago, the development of recombinant laminin-521 coating matrix replaced feeder cells for pluripotent stem cell (PSC) culture, which also enabled single-cell passaging. The natural signaling from full-length laminin, naturally present in the stem cell microenvironment, even obviated the need for apoptosis inhibitors.
The consistent support that Biolaminin offers even for single cells is one of the clear demonstrations of the importance of choosing a defined, biologically relevant matrix for various cell culture applications.
PSC single-cell survival after genome editing increased from 36 % to over 70 % by switching the substrate from vitronectin to Biolaminin 521
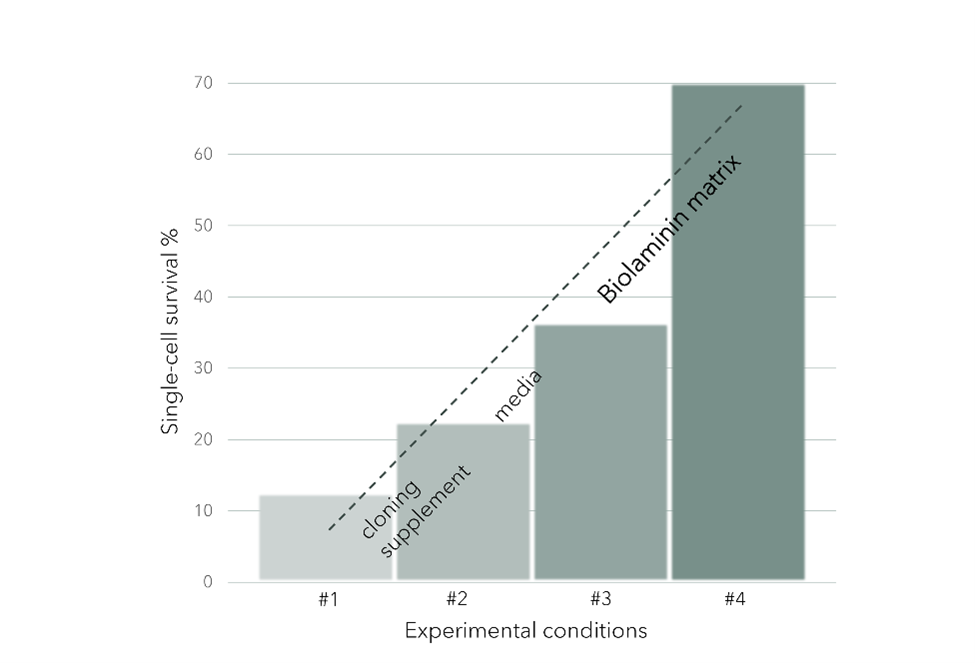
The study also shows high transfection efficiencies exceeding 97 %, and over 90 % single-cell survival after PSC sorting.

Talk to our team for customized support
We are here to help you in your journey.