Frequently Asked Questions
This FAQ page provides answers to common questions about Biolaminin and Biosilk products, including their properties, applications, and performance in cell culture. It also covers ordering, storage, and product compatibility. For further assistance, please do not hesitate to contact us.
About Biolaminin
-
Yes, Biolaminin is compatible with most surfaces, including glass, metals, dextran, and hydrogels. Laminin can be used to coat glass, providing good cell attachment and maintaining cell functions. Miyanari et al. (2013) recommended coating for 24-48 hours at +2°C to +8°C, with sealing of the coated glassware to prevent evaporation. Metzger et al. (2022) coated micropatterned glass cover slips with 10 µg/mL of recombinant Biolaminin 521, diluted in PBS+/+, for 3 hours at 37°C.
-
Basement membranes (BMs) are sheet-like extracellular matrix structures that provide the foundation for cell growth. The composition of the basement membrane (BM) is specific to certain cell types, and laminins play a key role in this. Laminins help cells attach to the BM and guide how they interact with their environment. In addition to their structural function in BM organization, laminins are crucial for regulating essential cellular processes such as adhesion, differentiation, migration, phenotype stability, and resistance to apoptosis. Without the right combination of laminin isoforms, cells and tissues can become dysfunctional.
Biorelevance emphasizes the importance of mimicking nature. Our laminin cell culture matrices allow you to replicate natural, cell-specific cell-matrix interactions in vitro, enhancing cell maturation, organization, and functionality. We offer a range of chemically defined, animal-origin-free, full-length laminin proteins for various applications, including reliable expansion of pluripotent stem cells and differentiation or maintenance of specialized cell types, such as hepatocytes, skeletal muscle cells, and neural cells. The effectiveness of our Biolaminin matrices in improving cell culture quality is supported by numerous high-quality publications.
-
✔ Enhanced consistency and reproducible results
✔ Improved cell identity and maturity
✔ Higher cell yield
The laminin cell culture substrates manufactured by BioLamina are the only full-length, recombinant laminins on the market. Full-length laminins preserve all functional domains essential for maintaining stable cellular identity, promoting long-term expansion, supporting efficient migration, and enabling single-cell passaging without the need for apoptosis inhibitors. In contrast, laminins isolated from tissues are impure mixtures of several ECM proteins and are degraded during isolation. Truncated recombinant laminin products, which contain only a small part of the protein, lack several essential interaction sites, preventing self-polymerization and hindering their ability to bridge the ECM with the cells, thereby compromising their function.
In summary, fragmented, truncated, and undefined tissue-derived laminins lack the critical domains necessary to form a proper extracellular matrix network and activate cellular signaling pathways. Only intact, recombinant full-length laminins can create a more authentic and defined cell culture environment. For further details, please refer to our Application Overview 008.
-
Biolaminin products differ in their intended applications and levels of quality control:
LN (research grade): Biolaminin LN is a full-length human recombinant laminin substrate. It is ideal for basic research. LN products are chemically defined, batch-to-batch consistent, and animal origin-free to the primary level.
MX (translational research grade): Biolaminin MX is also a full-length laminin substrate, but its production process is specifically designed for research aiming for translational applications, large-scale production, and regulatory compliance. MX products are chemically defined, batch-to-batch consistent, and animal-origin-free to the secondary level.
CTG (Cell Therapy Grade): Biolaminin CTG is a full-length laminin substrate developed for clinical applications. It undergoes more extensive quality control and regulatory compliance processes to meet the stringent requirements of clinical cell production for stem cell therapy and regenerative medicine. CTG products are chemically defined, batch-to-batch consistent, and animal-origin free to a secondary level, with comprehensive quality control documentation available.
For further information and a comparison of the three products, please refer to the Product sheet 006.
-
For optimal results, Biolaminin-coated plates should be used as freshly as possible. However, for convenience, coated plates with diluted coating solutions can be stored at 2–8°C for up to 4 weeks. Ensure that the surface does not dry out during storage.
-
Thawed Biolaminin stock solution (100 µg/mL) is stable for at least 3 months when stored at +2°C to +8°C under aseptic conditions. For long-term storage, it should be re-frozen at -30°C to -80°C.
-
Our data shows that Biolaminin 521 can undergo three freeze-thaw cycles without losing functionality.
-
Prepare the coating solution:
Dilute the thawed Biolaminin stock solution with 1x DPBS according to Instructions for Use 001.
Coat the cultureware:
Add the diluted Biolaminin solution to tissue culture-treated cultureware. Seal the cultureware (e.g., with Parafilm®) to prevent evaporation.
Incubation options:
Incubate at +2°C to +8°C overnight.
For faster coating, incubate at +37°C for 2 hours.
Cell culture:
Before seeding cells, remove the excess coating solution. No washing is required. Add the cell suspension directly to the Biolaminin-coated surface. For general PSC culture guidelines, refer to Instructions IN-003.
Storage of coated plates:
If not used immediately, store coated plates and any remaining diluted coating solution at +2°C to +8°C for up to 4 weeks.
Note: Do not allow the coated surface dry, as this will inactivate the laminin coating. -
Ca²⁺ and Mg²⁺ are divalent cations important for maintaining protein structure and function. When diluting Biolaminins, using DPBS with Ca²⁺/Mg²⁺ is preferable. However, based on our in-house experience, Biolaminins are stable proteins and can also be diluted in DPBS without Ca²⁺/Mg²⁺. The absence of these cations does not affect coating efficiency or protein functionality.
-
Here’s a list of primers for the analysis of tissue and cell-specific gene expression of laminin chains.
GenBank accession no. 5′ primer 3′ primer Amplicon size, bp Annealing temp, °C LAMA1 NM_005559 GTCAGCGACTCAGAGTGTTTG AACTTGGGTGAAAGATCGTCAG 185 55 LAMA2 NM_000426 GAACCCGCAGTGTCGAATCT GGGGAGTTAGCTGCCTTCA 204 55 LAMA3 NM_000227 TAGACTTTGGAAGCACCTACTCA GTTTATCAAGGACACCACAACCT 185 55 LAMA4 NM_002290 GCAGTGGAAATTCAGATCCCA TAACCGCAGGTCATCAGTCAG 275 55 LAMA5 NM_005560 GGTGTGTCTCTGCGTGACAA CCCCGACGTAGAAGACGAA 253 55 LAMB1 NM_002291 AGGAACCCGAGTTCAGCTAC CACGTCGAGGTCACCGAAA 103 55 LAMB2 NM_002292 GCCCTGGGAACTTCGACTG GGAAGCACTTCTTTTCGTCCTG 227 55 LAMB3 NM_000228 TCCTCTTGTGTTTTGCCCTG CTGCCTGGAGTCACACTTG 206 55 LAMC1 NM_002293 TCGTCAACGCCGCCTTCAA GTGTCGGCCTGGTTGTTGTA 184 55 LAMC2 NM_005562 CCAGGAGGGAAGTCTGTGATT GCAGTGAATCCCATCAGTGTT 128 55 LAMC3 NM_006059 CCAGGTGCATCACATCCTGAG GACCCCATTTGGGCTCCATT 236 55 -
The molecular weights (MWs) of the different laminin isoforms are listed in the table below. These MWs are calculated based on the amino acid sequences of the laminin chains. However, it’s important to note that post-translational modifications, such as glycosylation, can affect the actual MW of the laminin molecules in their native form.
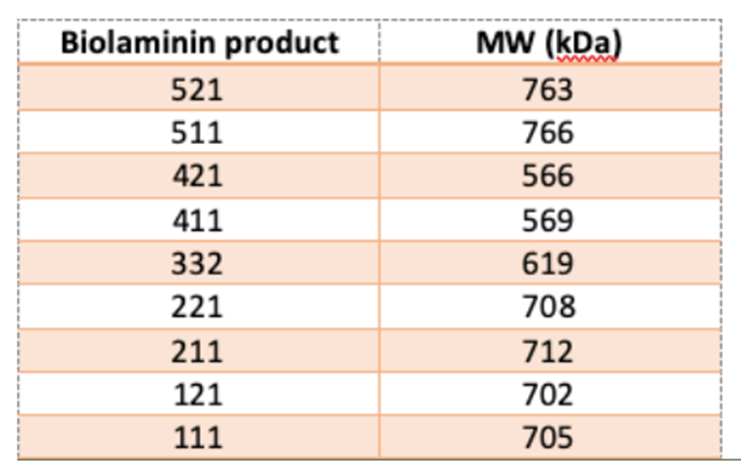
-
The LN332 trimeric protein has a total molecular weight of approximately 628 kDa (as determined by non-reducing SDS-PAGE) and is represented by three individual bands (~367 kDa, 130 kDa, and 131 kDa) in reducing SDS-PAGE. While we can confirm that full-length DNA was transfected, since the protein is naturally expressed, we cannot definitively confirm whether the LG4 and LG5 domains are cleaved during protein maturation. However, our Biolaminin 332 product has been shown to support enhanced cell attachment and growth, demonstrating its functional effectiveness.
-
Many of our customers successfully use the Biolaminin matrix for long-term culture protocols (lasting several months). The Biolaminin coating remains functional for at least one month in culture. For long-term cultures, if cell detachment is observed, we recommend adding 1-5 µg/mL of additional laminin to the medium (spiking the medium) to improve cell attachment.
-
Biolaminin is compatible with a variety of media. The robust support provided by the Biolaminin 521 culture substrate allows you to use your preferred medium and enzyme, making it easy to establish fully defined and animal-origin-free protocols.
-
Biolaminins work efficiently on a variety of plate types. Negatively charged, TC-treated plates (plasticware) provide optimal conditions for laminin binding and self-assembly. However, Biolaminin substrates can also be used to coat other materials, including glass, microcarriers, bioreactor hollow fibers, and more.
-
The absorption values for the Biolaminin proteins are displayed in the table below.
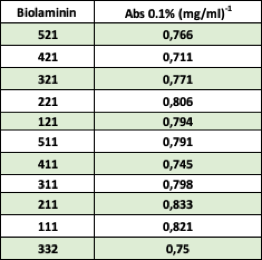
-
In vivo, basement membrane composition is highly cell-surface selective and for proper assembly, laminins are the key proteins. Laminin molecules self-assemble via a thermodynamically unfavorable nucleation binding followed by a calcium-dependent polymerization of the LN domains in the short-arms of the α, β, and γ chains (Yurchenco et al., 1985; Carafoli et al., 2012; Yurchenco & Cheng, 1993; Purvis & Hohenester, 2012). The sheet-like laminin network binds to other proteins in the basement membrane. Laminin interacts with nidogen via LE motifs of the γ1 and γ3 chains (Gersdorff et al., 2005; Takagi et al., 2003; Stetefeld et al., 1996), and the Lβ domain of the β chains binds to agrin (Domogatskaya et al., 2012). Laminin is linked to collagen IV through nidogen and heparin interactions, forming a covalently stabilized network (Hohenester & Yurchenco, 2013).
Laminins are large proteins with both hydrophobic and hydrophilic sites that allow them to interact with artificial surfaces. The highest density of anionic (negative) charges in laminins is to be found in the LG modules. It has been suggested that the density of negative charges on the surface could be critical for laminin-binding and self-assembly. Tissue-culture treatment is a process by which polystyrene surfaces are made to become hydrophilic, usually by increasing negative charge through a chemical means. This increased negative charge is important for cell attachment and may explain why cells cultured in non-treated plastic ware clump up since they are not attaching adequately to the surface.
The net charge and isoelectric pH (pI) of the Biolaminin proteins are displayed in the table.
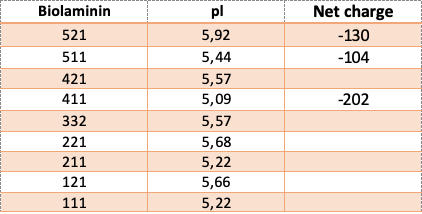
-
As a tool to identify and study the expression pattern of different laminin subunits present in a specific tissue, we recommend using the Laminin Marker Panel of PrecisA Monoclonals developed in collaboration with Atlas Antibodies.
-
The α4 and α5 chains of our Biolaminin LN products (i.e. LN521, LN511, LN421, LN411) have a FLAG-tag at the N-terminal end. The α2 chain of LN211 has an HA-tag at the N-terminal end and a FLAG-tag at the C-terminal end. The LN111, LN121, LN221, and LN332 protein products do not contain any tags. Similarly, the Cell Therapy grade Biolaminin 521 (CT521) and the MX521 do not contain tags.
-
The frozen Biolaminin stock solution has long-term stability when stored at -20°C to -80°C. For your convenience, you can aliquot the Biolaminin stock solution into smaller aliquots and store it at -30°C to -80°C. Repeated freeze/thaw should be avoided. Please refer to the product-specific CoA.
Thawed Biolaminin stock is stable for at least 3 months when stored at +2°C to +8°C under aseptic conditions. Avoid long exposure of the protein to ambient temperatures.
About Biosilk
-
There are significant differences in cell adhesion, spreading, and expansion between Biosilk and hydrogels.
Biosilk forms a fibrous network that mimics the dimensions of collagen fibers in real tissue, enabling cells to establish firm cell-matrix contacts (focal adhesion points) and migrate along the fibers to form cell-cell contacts (Sozzi et al. 2022; Johansson et al., 2019). This provides a more tissue-like environment, improving physiological cell morphology and promoting more cell-to-cell interactions compared to a hydrogel.
The network structure of Biosilk also facilitates efficient diffusion of media and oxygen, ensuring there are no necrotic centers (Fiorenzano et al. 2021, Sozzi et al. 2022).
Additionally, Biosilk allows for customized protocols for tissue- or cell-specific applications, particularly when combined with biorelevant Biolaminin isoforms, and supports long-term cultures with minimal handling (over 6 months, Fiorenzano et al. 2021).
.
-
Biosilk is a recombinant fragment of spider silk protein. It is engineered to form a robust, fibrous network that mimics the structure of natural extracellular matrices, providing a versatile scaffold for cell culture and tissue engineering. Due to its biocompatibility, biodegradability, and ability to support cell adhesion, migration, and differentiation, Biosilk is widely applicable to 3D cell culture and various biomedical applications.
For more details on its properties, recommended use, and supporting data, please visit the Biosilk product page.
Contact us for more information.
-
No, Biosilk is not a gel product. Instead, it forms a fibrous network. Biosilk is a recombinantly produced spider silk protein that can be shaped into various 3D structures. It can also be combined with different ECM proteins, such as laminins.
-
Biosilk 521 is Biosilk functionalized with Biolaminin 521, a full-length human recombinant laminin-521, which is a key cell adhesion protein of the natural stem cell niche. This addition provides Biosilk 521 with unique properties that promote the integration, proliferation, and lineage-specific differentiation of human pluripotent stem cells in a 3D culture format. For more details, please refer to the Biosilk product page.
-
Biosilk foams are created by introducing air bubbles into a Biosilk solution, forming a foam with thin Biosilk membranes surrounding each air bubble. Over time, these membranes burst and curl into thin fibers, creating a 3D network in place of the bubbles. The transformation from foam to network is influenced by cell density and growth. Typically, smaller bubbles merge into larger ones, which disappear within 1-4 days, leaving behind a multi-layered 3D network with cells uniformly integrated between the microfibers.
-
Yes. For free-floating 3D Biosilk cultures, 96-well plates coated with Ultra Low Attachment (ULA) surface are ideal, with 5 or 10 µL of Biosilk per well. This setup allows the 3D Biosilk cultures to reach approximately 300 µm in size (depending on the cell type) and allows easy handling and media changes. For detailed instructions, please refer to Instructions for Use 012.
-
For 96-well plates, a final volume as low as 5 µL, including cells, is feasible. Ensure that the Biosilk solution is not diluted below 2 mg/mL. For foaming, set the pipette to twice the volume of the Biosilk solution (e.g., 10 µL for 5 µL of foam). For further details, please refer to the 96-well plate guide.
-
Biosilk is a recombinantly produced protein, making it a defined and animal-origin-free product. Additionally, Biosilk 521, which contains Biolaminin 521, is also animal-origin-free and defined, as Biolaminin 521 itself is a fully defined, recombinant protein.
-
It is possible to add a hydrogel to Biosilk after the network has formed (i.e., after the bubbles in the foam are gone). However, embedding the organoid in Matrigel is not necessary to maintain its shape and cell phenotype. If embedding is preferred, it is recommended to use a xeno-free and defined material, such as HyStem™ (available from Merck).
-
Yes, you can mix Biosilk with any Biolaminin product. To do so, add 25 μL of the Biolaminin product of your choice to the thawed Biosilk solution (250 μL). Mix gently by pipetting 3 times without introducing air bubbles. The final mixture will have a concentration of 9 µg/mL Biolaminin.
-
Biosilk 521 has a Biolaminin 521 concentration of 9 µg/mL. The Biosilk 521 solution is prepared by blending 10 volumes of Biosilk with 1 volume of Biolaminin 521, resulting in a 10:1 ratio. This concentration is optimized for the culture of pluripotent stem cells (PSCs).
-
In its liquid form, the soluble Biosilk protein begins to unfold at 30°C (Hedhammar et al. 2008). However, once transformed into silk fibers, such as by foaming, Biosilk fibers are thermally stable up to 267°C and can withstand autoclaving (Hedhammar et al. 2010).
-
Biosilk is a stable protein in vitro once transformed into silk fibers (Johansson et al. 2019), and can sustain long-term organoid cultures for over 6 months (Fiorenzano et al. 2021).
-
The molecular weight of Biosilk protein is 25 kDa before polymerization.
-
Biosilk’s autofluorescence is negligible in the blue and far-red channels. However, depending on the thickness of the Biosilk scaffold, some autofluorescence may be observed in the green channel. If necessary, autofluorescence can be suppressed using Sudan Black. For more technical details, refer to Neo et al. 2015 (Tissue Eng Part C Methods).
-
Foam detachment may occur due to one or more of the following reasons:
- Non-hydrophobic surface: If the surface where the foam is placed is not hydrophobic, detachment can occur. Culture plates intended for suspension culture or non-tissue treated (non-TC) plates are typically hydrophobic. We recommend using plates from SARSTEDT, ref: 83.3922.500.
- Small foam diameter: If the foam’s diameter is too small compared to its height, the contact area with the bottom may be insufficient. This can cause the foam to lift when medium is added. For a 20 µL foam, a diameter of 0.7-1 cm is optimal for good attachment.
- Extended standing of Biosilk solution: If the Biosilk solution has been left at room temperature too long after thawing (more than 45-60 minutes), it may cause protein precipitation, resulting in a milky appearance. The solution should be used immediately after thawing.
- Abrupt medium addition: Adding medium with excessive force can break or lift the foam. It is important to carefully first drop the medium around and then on top of the foam to prevent disruption.
-
The foam may break into pieces for one of these reasons:
- Extended time at RT: If the Biosilk solution has been left at room temperature too long after thawing (more than 1 hour), the protein may partially precipitate, causing a milky appearance. The solution should be used immediately after thawing.
- Improper stabilization process: Ensure the stabilization process is carried out according to the protocol at 37°C to maintain foam integrity.
- Abrupt medium addition: Adding medium with excessive force can disrupt or lift the foam. Carefully drop the medium around and then on top of the stabilized foam to avoid breaking it.
- Excessive dilution of Biosilk: The final concentration of Biosilk should not be lower than 2 mg/mL. If adding another Biolaminin isoform to the Biosilk, ensure the total added Biolaminin volume does not exceed 1/10 of the Biosilk volume.
- Improper pipetting: When creating the foam, pipet 15-25 times rapidly within 10-15 seconds to generate small, evenly distributed bubbles. Avoid creating overly large bubbles, as they can create holes. Once foaming is complete, do not continue pipetting, as this can disrupt the 3D structure.
-
For optimal and even integration throughout the 3D Biosilk, cells should be added either before or directly after the foam is created. Please refer to our Instructions for Use 011 for further details. It is also possible to seed cells onto a premade Biosilk foam; however, this results in less uniform distribution throughout the 3D structure (Johansson et al., 2015). Note that for the creation of Biosilk membranes, cells can be seeded after the formation process (See Gustafsson L et al., 2024).
-
Yes, most proteins will have a tendency to get entrapped/entangled in the Biosilk.
-
Make sure a hydrophobic culture plate is being used (e.g. Sarstedt 83.3922.500). If the plate is not hydrophobic, the Biosilk solution will be difficult to pipette and the foam will become flat. Plates that are intended for suspension culture or are non-tissue treated (non-TC) are often hydrophobic.
Animal Cells
-
Yes, Biolaminins are full-length laminins capable of binding to other ECM components, such as collagens (a property that fragmented and truncated laminin products lack). Biolaminin can be used in combination with other proteins for specific experimental needs. Biolaminin is also commonly employed for biofunctionalization to enhance cell attachment, migration, and differentiation. For more details on the biofunctionalization of hydrogels, please refer to Application Note 024.
-
No significant difference in cell survival or proliferation rate has been observed between the slow and fast coating when following the recommended coating protocol. For detailed guidance on preparing and using the Biolaminin 521 coating solution, please refer to Instructions For Use 001.
-
Yes, laminins, like many other basement membrane proteins, are highly conserved across species. Biolaminin provides a biologically relevant substrate for stem cell cultures across different species. Many customers working with animal stem cells from species such as mouse, sheep, rabbit, horse, feline, bovine, porcine, and monkey are successfully using our human recombinant laminins.
-
The issue could be caused by one or more of the following factors:
- Coating concentration too low: The coating concentration may be insufficient for your specific cell line. Try increasing it to 10 μg/mL. Once the cells are adapted to the Biolaminin 521 matrix, you can attempt to reduce the coating concentration to 5 μg/mL.
- Insufficient coating volume: This issue is often seen in smaller well sizes due to surface tension. For 24-well plates, 500 μL of coating solution per well should be sufficient for even coverage. For 6-well plates, at least 1 mL should be used.
- Coating solution drying before cell seeding: If the coating dries out before cell seeding, it will inactivate and fail to support cell growth. This can happen if the coating volume is inadequate or if there is too much time between the exchange of coating solution and culture medium. To prevent this, seal the plate during overnight coating in the fridge. If using a quick coating at 37°C, do not leave the plate for more than 3 hours to avoid drying out.
- Uneven cell seeding: Ensure that the cells are evenly distributed during the seeding process. Gently rock the plate side-to-side after seeding to achieve an even cell spread.
- Issue with cultureware: The problem may also lie with the cultureware itself. Biolaminin coating has been tested and shown to work well on tissue culture plates from brands such as Falcon, Sarstedt, and Corning.
-
No, Biolaminin 511 and 521 can support the self-renewal of mouse pluripotent stem cells (mPSCs) in the absence of feeder cells, LIF, or other differentiation inhibitors, even at low cell density. Both laminin isoforms have been shown to successfully support both naive and primed stem cells, as they activate the PI3/Akt pathway while leaving the MAPK/ERK pathway unaffected (Rodin et al., 2014; Domogatskaya et al., 2008).
-
We recommend two coating protocols: a 2-hour incubation at 37°C, or a slower coating with incubation at +4°C overnight. If you wish to optimize your protocol further, a titration experiment is recommended to adapt the Biolaminin concentration and coating time to your specific cell culture needs. For more detailed instructions, please refer to Instructions for Use 001.
-
For culturing hESC, iPSC lines, and MSCs, a starting Biolaminin 521 concentration of 10 µg/mL is recommended. Once the cells are adapted to the Biolaminin 521 coating, the concentration can generally be reduced to 5 µg/mL. The optimal coating concentration is cell type-dependent and should be optimized empirically for specific isoforms and cell lines. For certain specialized cells, such as cardiomyocytes, a higher coating concentration (10 µg/mL) may be required. Using too low a coating concentration could lead to slow growth or uneven cell spreading.
-
In-house, we typically use functional cell attachment to estimate coating optimization. By seeding the same number of cells onto plates coated with different amounts of Biolaminin and measuring cell confluence the next day, we can identify the optimized coating concentration for the tested cell line. The optimal coating concentration is reached when most cells attach, and the monitored confluence (%) no longer increases with higher coating concentrations. The optimal concentration may vary between cell lines, but we generally recommend starting with 10 µg/mL and decreasing to 5 µg/mL once the cells have adapted to the Biolaminin culture conditions.
Another method to assess coating efficiency is ELISA. One of our customers used an ELISA method where they coated plates with varying amounts of Biolaminin and employed an antibody (Abcam ab11575, rabbit polyclonal against mouse EHS) to detect and measure the laminin protein. This antibody recognizes various laminin chains and can be used for this general purpose. If different laminin isoforms are used for coating, monoclonal antibodies specific to each laminin chain are available from Atlas Antibodies (Laminins Marker Panel), allowing for the separation and identification of individual isoforms.
-
Yes, it is possible to release cells from the Biosilk network through enzymatic detachment, using reagents like trypsin or Accutase. The Biosilk fibers are highly stable against proteases in their silk form and will not degrade during this process. For long-term culture in tissue-like organoids, a cell dissociation protocol for tissues may be required. If a single-cell dispersion is needed for subsequent analyses, such as flow cytometry, the remaining Biosilk fibers can be removed using a cell strainer.
-
Yes, you can seed your preferred stem cells in, before or after foaming the Biosilk, and then culture them in expansion media until the desired density is reached, before switching to differentiation media.
Depending on your desired 3D culture size, you can use either 96-well or 24-well plates:
Floating 3D cultures:
When using ULA-treated 96-well plates, add 5–10 µL of Biosilk per well to create floating 3D structures. For detailed instructions, see Application Note 012.Attached 3D cultures:
When using non-treated 24-well plates, add 10–20 µL of Biosilk per well to form attached 3D cultures. Once the cultures reach the desired density, they can be detached from the well surface and optionally cut into smaller pieces for downstream applications. Refer to Instruction For Use 011 for more details. -
When cells are added before foam formation, they are typically integrated evenly throughout the material. The 3D Biosilk foam is created by introducing air bubbles into the Biosilk solution for 10-15 seconds, a process that generally does not disrupt the cells. However, for highly sensitive cell types, it may be preferable to add the cell suspension directly to freshly formed foam, although this may result in slightly less uniform integration. It is important to avoid creating large air bubbles, as this can cause holes in the 3D Biosilk structure.
See also our Instructions for Use 011.
-
The optimal coating concentration depends on the cell type. Typically, the ideal range is between 0.5 µg and 3 µg/cm²; please refer to our Coating Instructions 001 for further guidance. After a few passages, the concentration can often be reduced, making Biolaminin culturing more cost-effective. A concentration that is too low may result in poor cell attachment or detachment shortly after seeding.
Application
-
Yes, Biolaminin 521 supports the successful culture of pluripotent stem cells as single cells without the need for apoptosis inhibitors (Rodin et al., 2014). By recreating the biologically relevant hPSC milieu in vitro, Biolaminin 521 promotes authentic cell signaling that maintains stem cell identity and survival. For more information, please refer to Application Note 004.
-
The post-thaw cell survival is high when supported by Biolaminin 521. This is nicely described in a publication by Miyazaki and colleagues, where they show that hPSC that were frozen and thawed as colonies showed markedly decreased survival compared to cells frozen and thawed as single cells where the majority of the cells were viable (Miyazaki et al., 2013).
-
Optimal seeding densities can vary depending on the specific cell line and should be determined empirically for your system. Biolaminin 521 has been shown to support cell survival at densities as low as 5,000 cells/cm². However, we generally recommend seeding at a density of 30,000–50,000 cells/cm² or splitting your cells at a ratio of 1:10 to 1:30 (Rodin et al., 2014). Biolaminin 521 also supports single-cell expansion, such as during gene editing. For detailed guidance, please refer to our application notes:
Application Note 004: Expansion and maintenance of pluripotent stem cells
-
Yes, Biolaminin 521 supports both single-cell and clump passaging of stem cells. However, we recommend single-cell passaging or passaging small clumps, as it is a more reliable method that allows for standardization. This approach ensures that each cell has equal contact with the coating and medium, promoting a homogeneous environment. Biolaminin 521 serves as the natural niche protein for the cells and facilitates cell-cell contact by promoting high cell migration. The survival rate after single-cell seeding is high, and no apoptosis inhibitors, such as Rho-kinase (ROCK) inhibitor or blebbistatin, are needed. Consequently, the conventional method of maintaining the colony state to prevent apoptosis after re-seeding is unnecessary. Single-cell passaging also reduces the risk of spontaneous differentiation.
-
We do not recommend reusing the coating solution, as doing so may affect the consistency and quality of the coating. Instead, we suggest evaluating the optimal coating concentration for your cells and application through titration. Often, a lower concentration than the recommended starting concentration can be used. For Biolaminin 521-adapted hESC or iPSC lines, a coating concentration of 5 µg/mL typically works well. For many MSC lines and neural applications, a coating concentration as low as 1 µg/mL can be effective. The optimal coating concentration is cell type-dependent and should be empirically optimized for specific isoforms and cell lines.
-
Although not yet available as official protocols, several scientific articles have demonstrated how Biosilk can be used to create membranes that mimic the basement membrane in setups similar to Transwells. Biosilk membranes enable biomimicry, customization, and biodegradability, making them ideal for long-term, physiologically relevant setups.
A general procedure and characterization of the resulting membrane are described by Gustafsson and colleagues (Gustafsson et al. 2020, Advanced Functional Material), with a complementary video description in Gustafsson et al. 2024 (J Vis Exp). In brief, nanofibrillar Biosilk membranes are formed by allowing a 1 mg/mL Biosilk solution to stand still for 8 hours.
Tasiopoulos et al. 2021 (ACS Biomater Sci Eng) detail how a Biosilk membrane can be utilized to model a blood vessel wall, while Hjelm et al. 2023 (Biochem Biophys Res Commun) explore its use in modeling the blood-brain barrier.
-
Yes, it is possible. For further details, refer to the following publications: Johansson et al. 2019 (Scientific Reports), Åstrand et al. 2020 (Biomaterials Science), Collodet et al. 2023 (Bioengineering & Translational Medicine), Fiorenzano et al. 2021 Nature Communications, Sozzi et al. 2022 (Frontiers in Cell and Developmental Biology), Gkouma et al. 2024 (Biofabrication).
Please note that Biosilk has some autofluorescence in the green channel. For more information, see other FAQs.
-
See this reference for technical details about Sudan Black (SB) treatment: Neo et al. 2015 Tissue Eng Part C Methods.
In this study, SB was incorporated into fluorescence-based staining protocols and found to significantly reduce the intrinsic fluorescence of silk, particularly improving imaging in the blue and green emission wavelengths.
Cell culture
-
You can use a dissociation reagent of your choice, such as TrypLE Select, Trypsin-EDTA, EDTA, or Accutase. The incubation time will vary depending on the dissociation solution used and the specific cell line.
-
Within 1 hour after seeding on LN521, the majority of the cells should have attached and be evenly distributed across the surface. The cells show high motility and will migrate to establish contact with other cells, initiating proliferation.
Transfer from another matrix
-
We recommend transferring the cells as single cells (or small clumps) and always adding ROCKi for the first 3-5 passages. Once the cells are adapted to the Biolaminin matrix, they can be cultured as single cells without the need for ROCKi. For further details, please refer to Instruction for Use 003.
-
Cells grown on other feeder-free substrates, such as Matrigel or vitronectin, can generally be transferred directly onto Biolaminin 521 without the need for specific adaptation. Pluripotent stem cells will maintain their pluripotent characteristics. However, there may be small morphological changes when switching substrates. For further details, please refer to Instructions for Use 003.
-
If the transfer from other culture substrates is problematic, please follow the Transition Guide in Instructions for Use 003. Here are the main recommendations:
- Only high-quality cells should be transferred to the Biolaminin 521 matrix. Carefully select only undifferentiated areas for transfer.
- Avoid changing the substrate and media at the same time. If you are also changing the medium, transition to the Biolaminin 521 matrix first before adjusting the medium.
- For initial passages, use a higher coating concentration of 10 µg/mL for LN521 and 20 µg/mL for MX521 and CT521 to support cell attachment and adaptation.
- Seed at a higher cell density (50,000-100,000 cells/cm²) for the first few passages. This might be influenced by the culture medium. Different cell densities can be tested in parallel to determine the optimal conditions.
- Include ROCK inhibitor (10 µM) during the initial passages to support cell survival and adaptation. Parallel wells without ROCKi can be set up to assess whether the cell line adapts well without the inhibitor, allowing for a faster transition.
- Once cells adapt to the Biolaminin 521 matrix, the seeding density and coating concentration can typically be reduced, and cells can be routinely cultured as single cells without requiring ROCK inhibitor.
- When transitioning from Matrigel or another aggregate culture, the original culture can be maintained for 1–2 passages as a backup source.
- Transitioning from another single-cell culture typically does not require adaptation passages or ROCKi.
3D
-
Yes! Biosilk is an excellent 3D culture system for organoid cultures. Seed pluripotent stem cells (or another cell type of interest) either before or after transforming Biosilk 521 into a 3D scaffold by pipetting. Amplify the cells to the desired confluency before switching to your chosen differentiation medium. Once the cells have reached the desired confluency within the Biosilk network, the structure can be cut into smaller pieces (approximately 2 mm thick) using a blade or small scissors and transferred to low-attachment plates for culture as free-floating entities.
Other
-
In vivo, the composition of the basement membrane is highly selective to specific cell surfaces, with laminins playing a key role in its assembly. Laminin molecules self-assemble through a thermodynamically unfavorable nucleation binding, followed by calcium-dependent polymerization of the LN domains in the short arms of the α, β, and γ chains (Yurchenco et al., 1985; Carafoli et al., 2012; Yurchenco & Cheng, 1993; Purvis & Hohenester, 2012). This process results in the formation of a sheet-like laminin network that binds to other proteins in the basement membrane. Laminin interacts with nidogen via LE motifs on the γ1 and γ3 chains (Gersdorff et al., 2005; Takagi et al., 2003; Stetefeld et al., 1996), and the Lβ domain of the β chains binds to agrin (Domogatskaya et al., 2012). Additionally, laminin is linked to collagen IV through nidogen and heparin interactions, forming a covalently stabilized network (Hohenester & Yurchenco, 2013).
Laminins are large proteins with both hydrophobic and hydrophilic regions, enabling them to interact with artificial surfaces. The LG modules contain the highest density of anionic (negative) charges, which may be critical for laminin binding and self-assembly. Tissue-culture treatment involves modifying polystyrene surfaces to become hydrophilic, typically by increasing negative charge chemically. This increased negative charge is essential for cell attachment and may explain why cells cultured in non-treated plasticware tend to clump, as they are unable to adequately attach to the surface.
The net charge and isoelectric pH (pI) of Biolaminin proteins are displayed in the table.
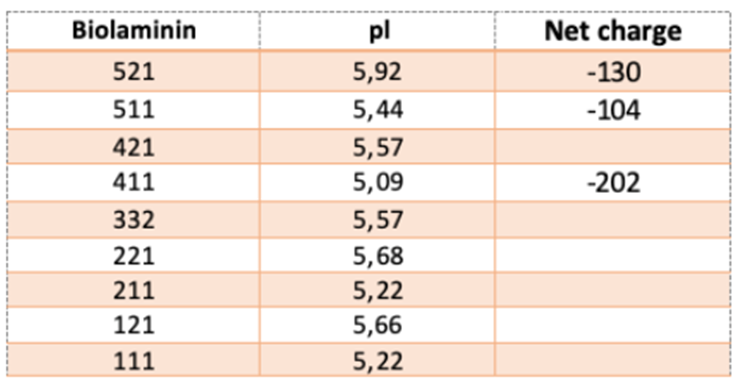

Do you have another question?
We are here to help you in your journey.