The matrix to trust for your stem cell therapy success
Successful pluripotent stem cell therapy requires reproducibility, global regulatory compliance, and most importantly, the generation of truly functional clinical cells. Biolaminin®, the only full-length laminin substrate, excels in supporting all these aspects.
Key advantages
Key advantages of Biolaminin in stem cell therapy production
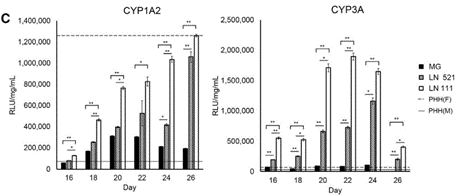
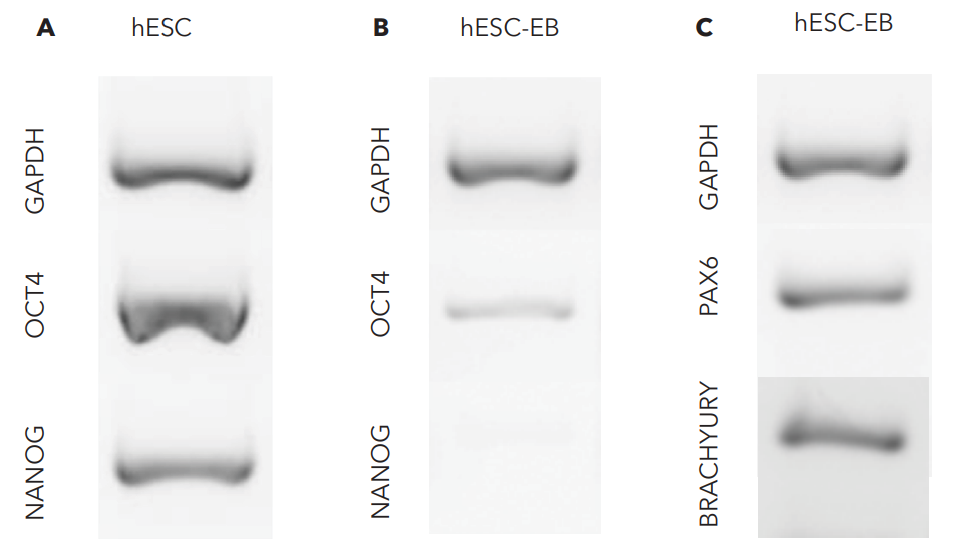
Full-length laminin proteins, Biolaminin, are scientifically proven to streamline differentiation, stabilize identity, and enhance cell growth across various culture platforms and conditions.
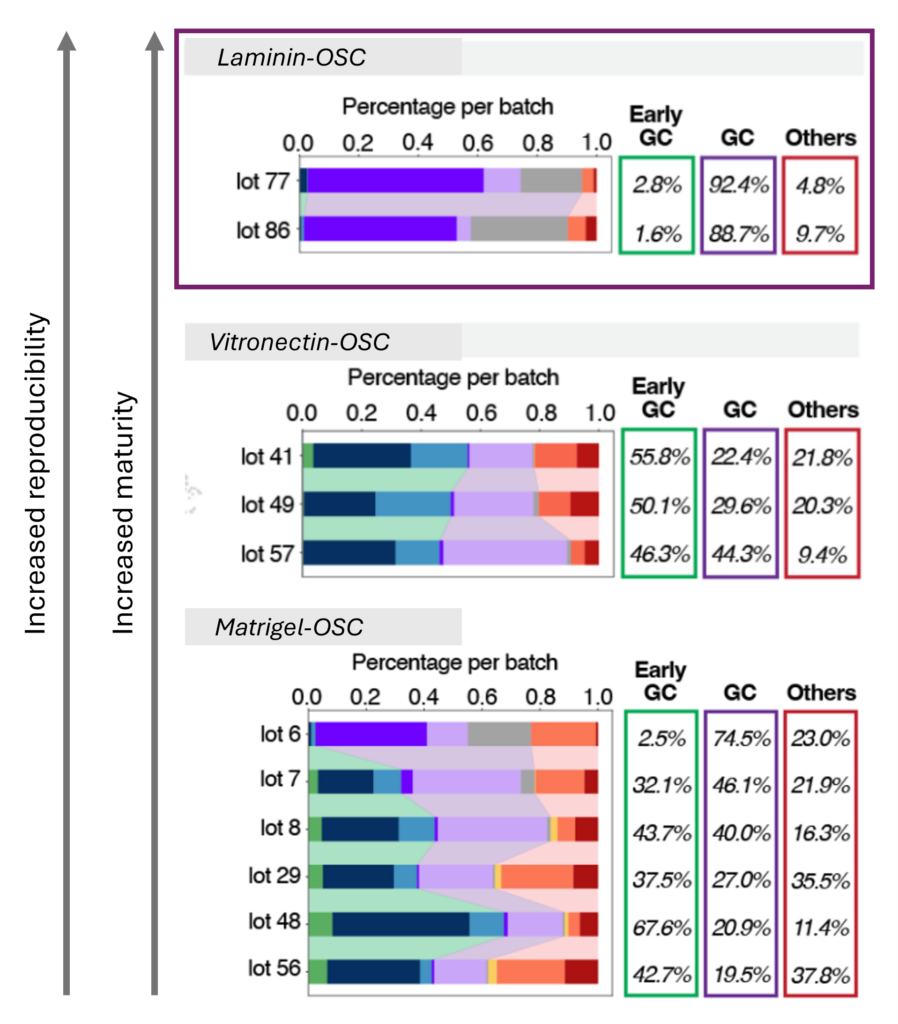
Reproducible and stable process
➞ Achieve consistently functional cell products— reliable every time
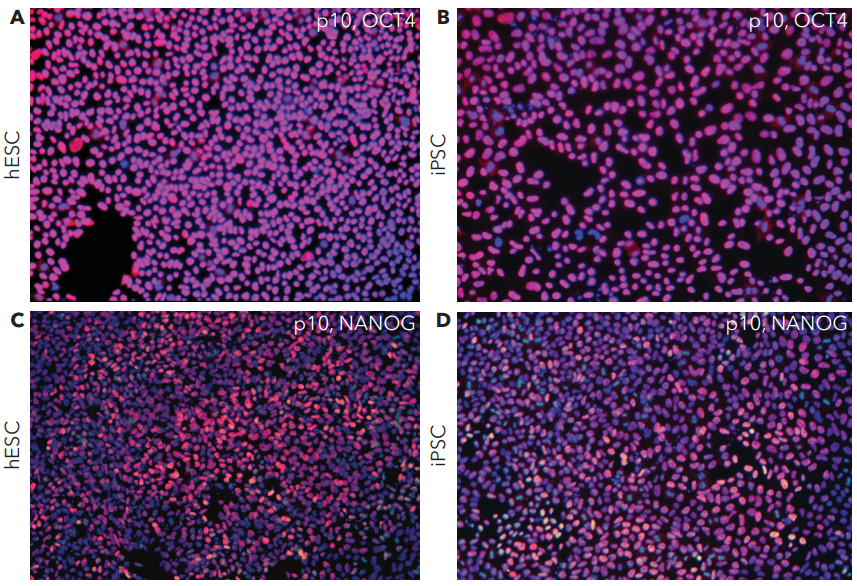
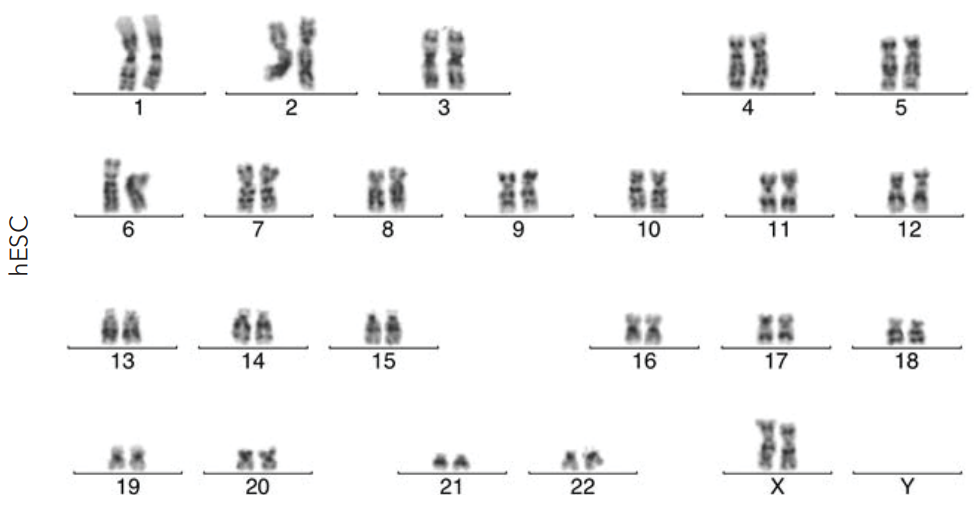
➞ Maintain stem cell pluripotency and cell identity
Reproducible and stable process
Maximized cell
yield
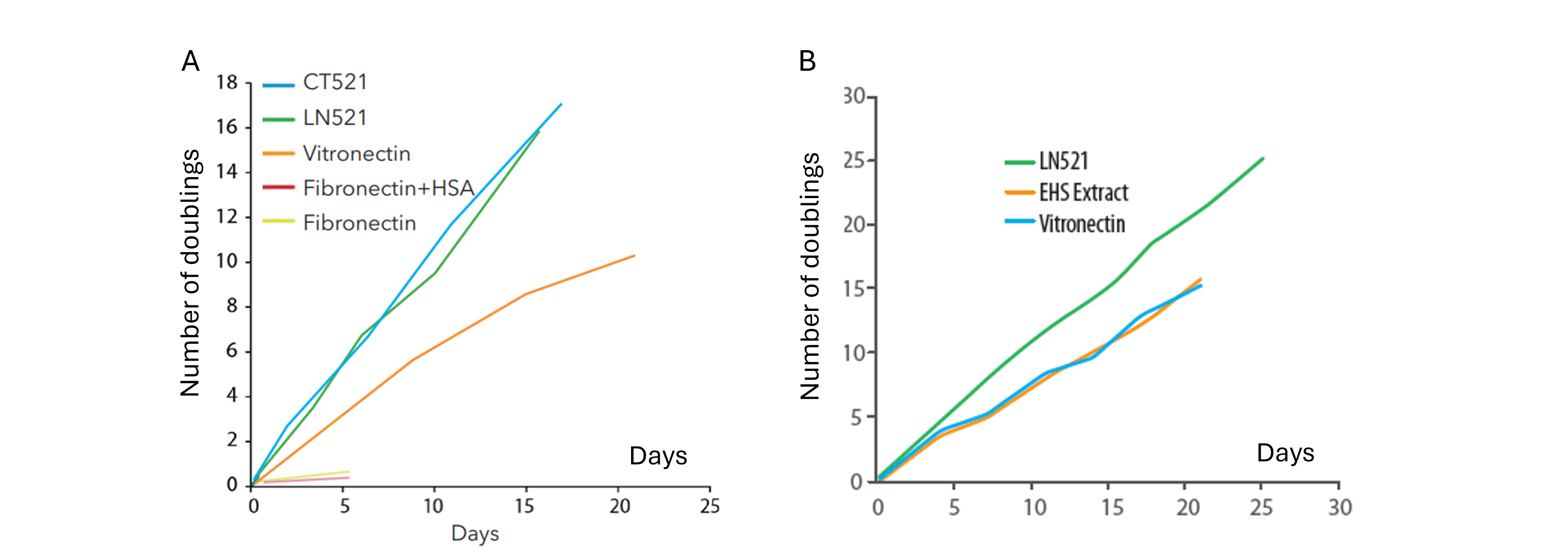
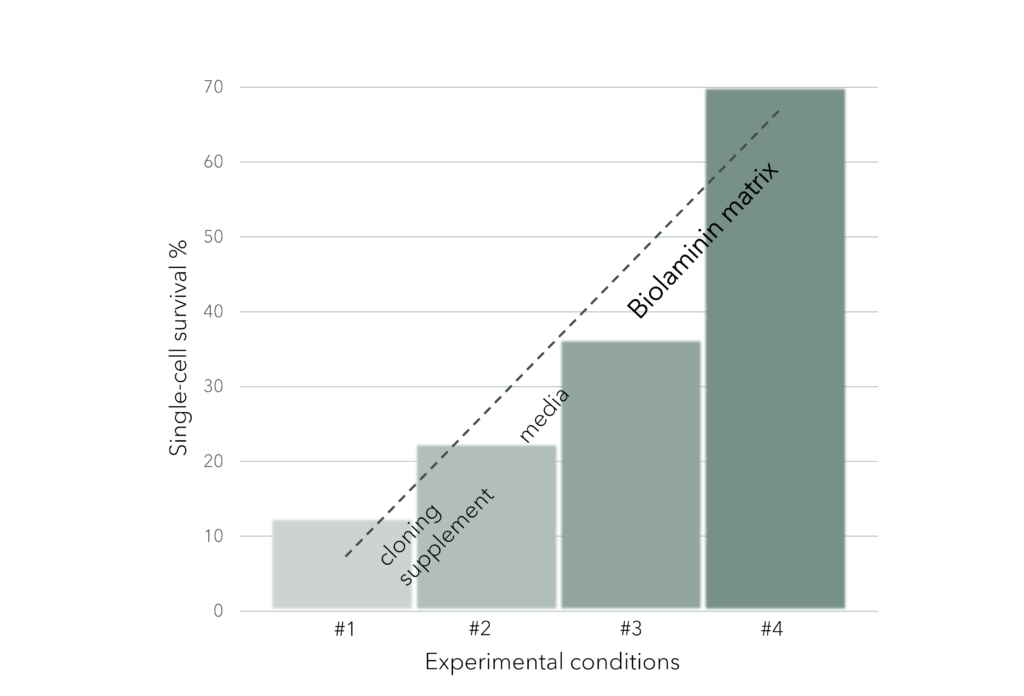
➞ Enhance cell proliferation and survival
➞ Optimize utilization of culture surface area
Maximized cell yield
Wide platform compatibility
➞ From single cells to large-scale expansion; applicable to various protocols with minimal adjustment
➞ From concept to standardized protocols and global regulatory compliance
Wide platform compatibility
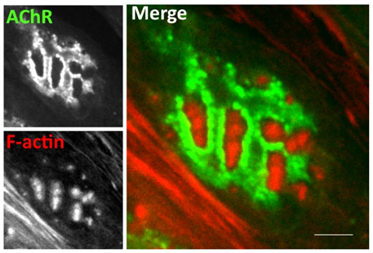
“We have struggled for a number of years to establish a GMP manufacturing process for generating DA neurons from human embryonic stem cells in order to develop a stem cell-based therapy for Parkinson’s Disease.
The recombinant laminins from BioLamina really made a world of difference!”

Prof. Malin Parmar
Lund University
What makes us unique
What makes us unique—biology is the key
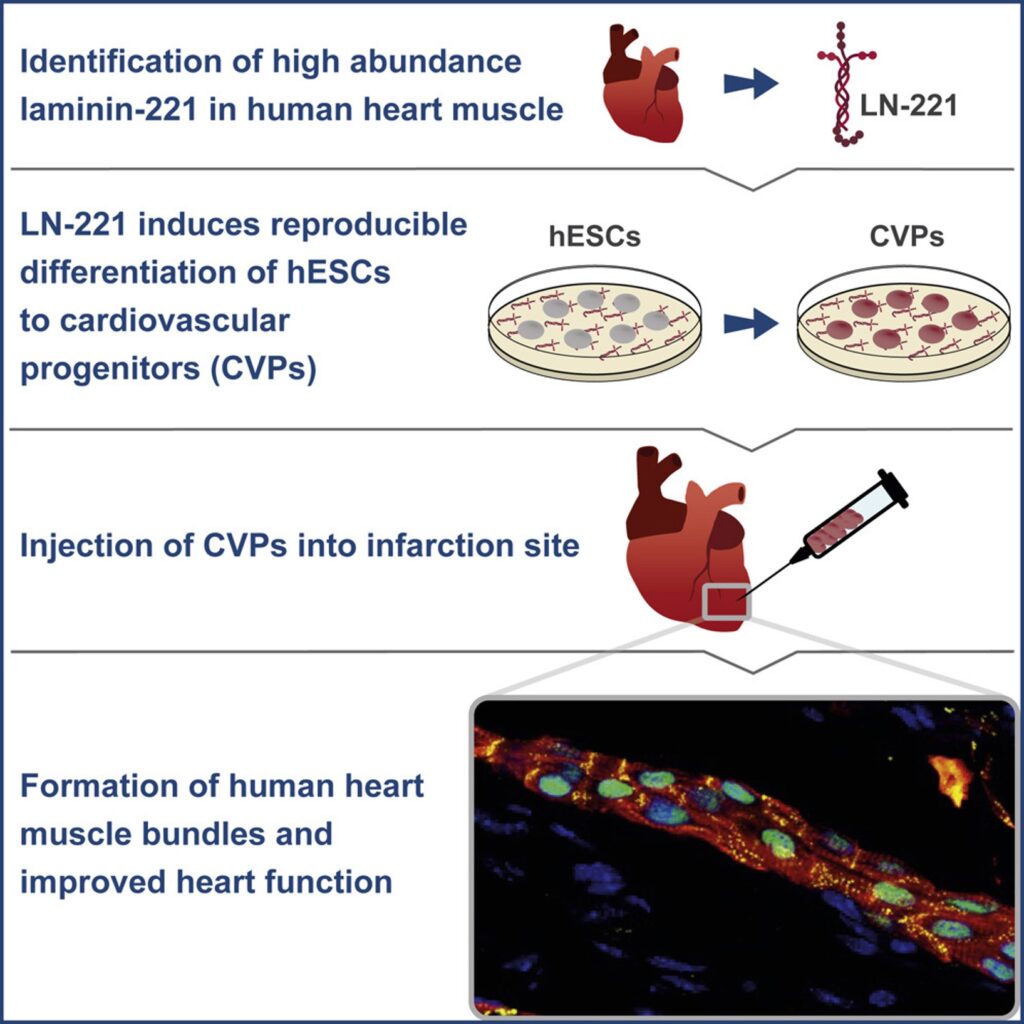
✓ The in vivo stem cell matrix
The key to vastly improved cell function lies in the essential roles of laminins within the stem cell microenvironment and across human tissues.
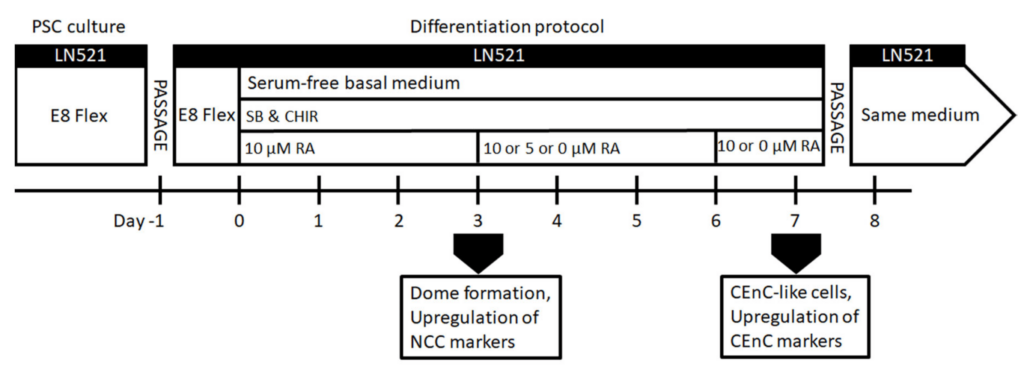
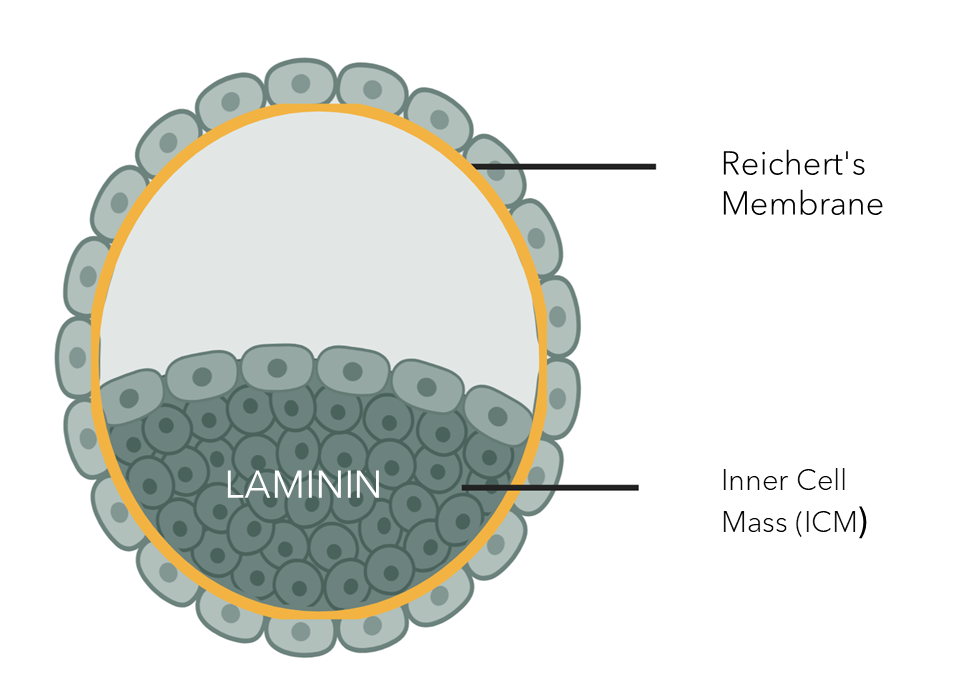
In the early embryo, laminin-521 is the first extracellular matrix (ECM) molecule to appear within the embryonic stem cell niche, already at the four-cell stage. Unlike other matrix products, full-length laminin serves as the foundational support for self-renewing stem cells in their natural niche.
✓ The only full-length laminin for authentic cell signaling
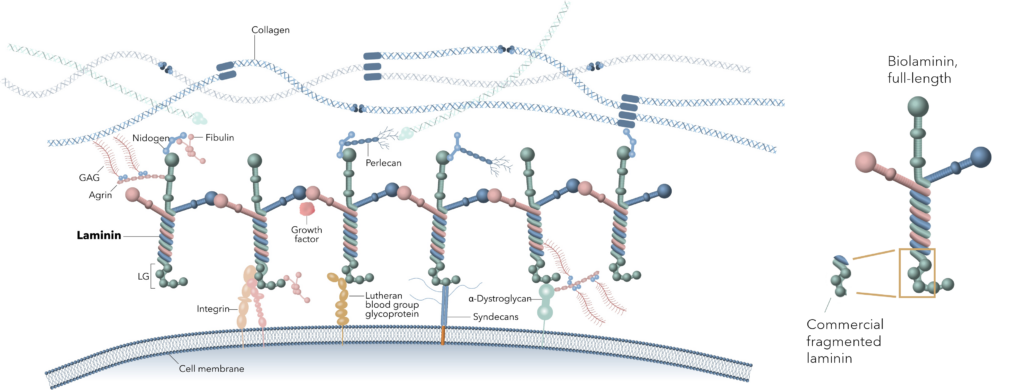
Laminin functions as a crucial link between the extracellular matrix (ECM) and cells, actively directing cell identity, proliferation, and survival through the activation of core signaling pathways.
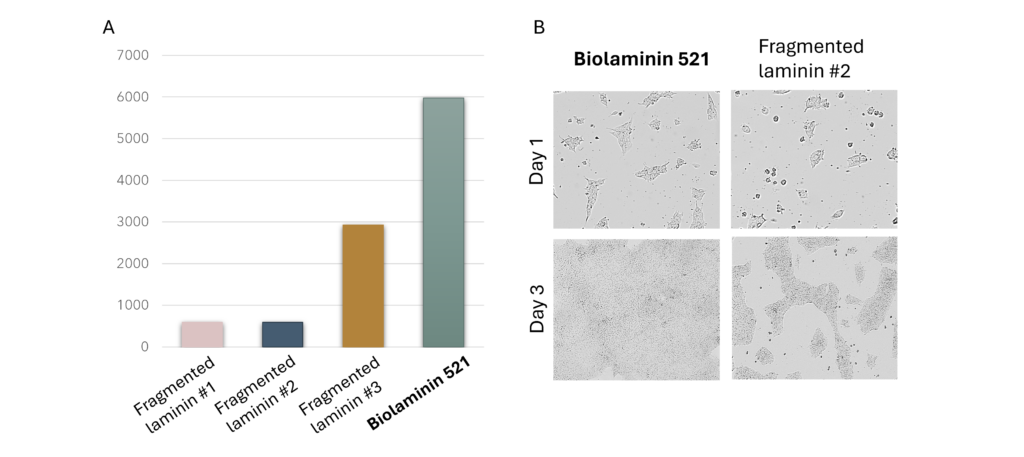
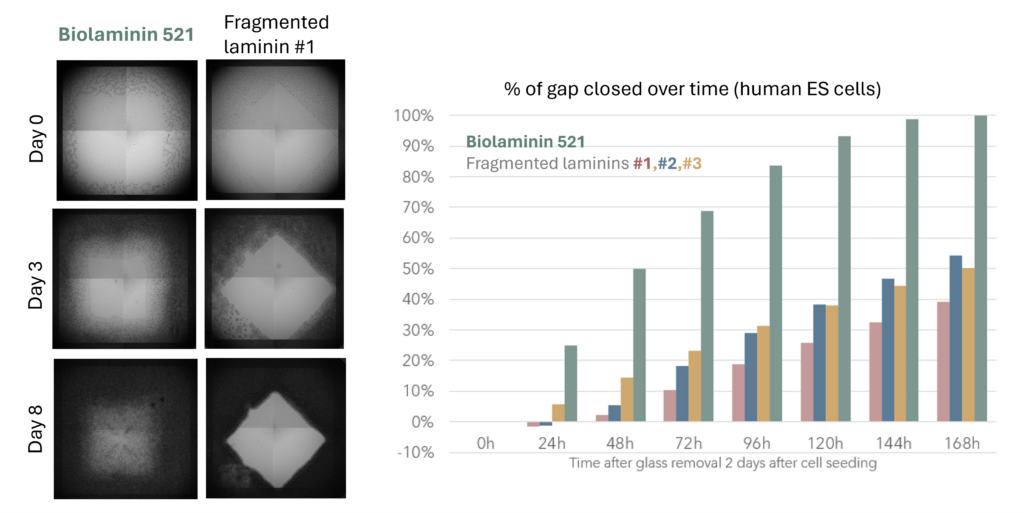
Unlike fragmented laminin products, full-length laminin can bind with all relevant cell surface receptors, including integrins, dystroglycans, and syndecans. Importantly, it is also able to interact with various microenvironmental factors, including growth factors.
✓ Tissue-specific laminin isoforms for specialized cell function
Laminin isoforms display diverse compositions across tissues, supporting cell specialization and functionality in a tissue-specific manner. Our exclusively full-length laminin products provide the largest laminin isoform portfolio on the market.
Our products
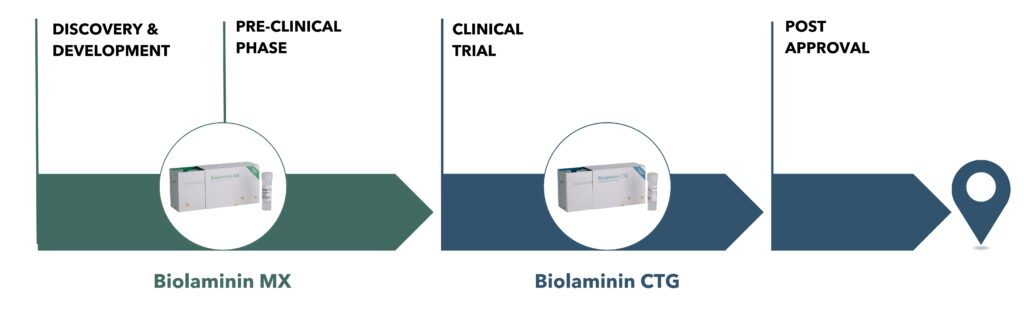
Take your stem cell therapy program from concept to clinic
Enhance your therapeutic cell production process with Biolaminin MX with a seamless transition to the cell therapy grade CTG product.
Explore our products
Optimize your differentiation process
Explore our tissue-specific laminin isoforms – tailored to your differentiation needs!
Read more about Biolaminin matrices and their applications
-
Application note 015: Cell therapy grade Biolaminin 521 CTG
Features of the Biolaminin CTG product designed for clinical use
Open pdf -
Product sheet: Biolaminin 521 cell culture substrates
See how our CT, and MX products differ
Open pdf -
Application note 022: BioLamina products in research and manufacturing – Biolaminin® matrices for all steps and scales
Clinically adaptable Biolaminin cell adhesion coating for higher yield in bioreactor systems
Open pdf -
Application note 018: Large-scale cell production
Clinically adaptable Biolaminin cell adhesion coating for higher yield in bioreactor systems
Open pdf -
Application note 020: Efficient gene editing on Biolaminin® 521 substrate
Efficient gene editing on Biolaminin® 521 substrate
Open pdf -
Application overview 008: Full-length laminin proteins are crucial for stem cells
Full-length laminins are crucial components in creating the stem cell niche in vivo and in vitro
Open pdf