Skin cells
Biorelevant culture of keratinocytes on Biolaminin substrates
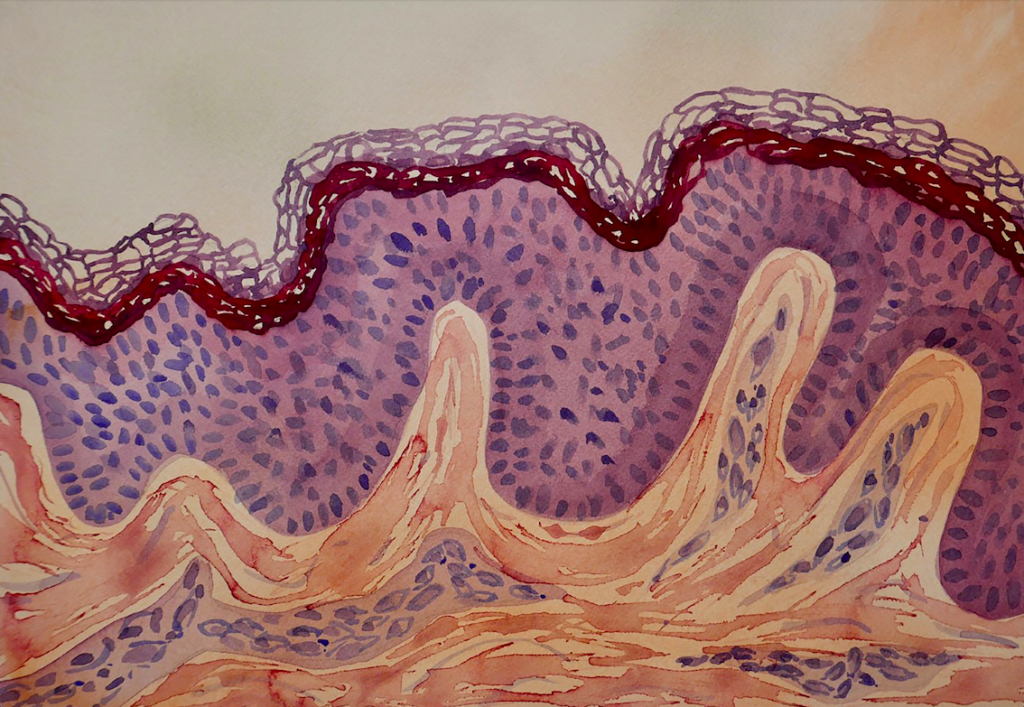
The basement membrane composition in the epidermis
Human epidermal keratinocyte (HEK) cells are the major cell type of the epidermis, the outermost layer of the skin. HEK cells maintain the barrier function of the skin and make up a major component in the wound healing process.
The keratinocytes arise as basal cells at the innermost layer of the epidermis where they are positioned on a specialized basement membrane (BM) composed of a set of distinct proteins, type iv, and xviii collagens, perlecan, agrin proteoglycans, as well as laminin (LN) proteins. Following cell division, they migrate into the upper epidermal layers, ultimately terminally differentiate to form the dead surface layer of the skin (Eckert and Rorke, 1989).
A feeder-free, xeno-free and fully defined method to culture human epidermal kerotinocytes
The skin-specific laminin proteins play important roles in the maintenance of keratinocyte phenotypic integrity and promote cell survival. The BM underlying the neonatal and adult epidermis is highly enriched in the a5 laminin isoforms (LN511 and LN521). Pouliot and colleagues demonstrated within an in vitro cell adhesion wound-healing assay that the human recombinant laminin cell culture substrates Biolaminin 511 (LN511) and Biolaminin 521 (LN521) are both potent adhesive substrates for both neonatal and adult keratinocytes and that this cell-matrix adhesion is mediated by the α3β1 and α6β4 integrins (Pouliot, 2002).
In a recent Nature Communications article, LN511 was shown to enable robust, feeder-free, and completely xeno-free and defined long-term expansion of primary adult human dermal keratinocytes. This culture system is comparable to the 3T3-J2 feeder co-culture system in terms of colony-forming efficiency, basal marker profile, genetic stability, and the ability to form a normal stratified epidermal structure in both in vitro and in vivo models (Tjin, 2018). LN511 cultivated HEK cells also demonstrate a significantly reduced pro-inflammatory signaling pathway expressions compared to classical feeder culture-based systems.
This ground-breaking article has been followed up by an article in Nature Protocols (Tjin, 2020) where the authors show that it only takes between 7 and 14 days to obtain an initial culture and once the cells reach confluency and that a secondary culture from the primary culture can be expanded up to 20-fold within 4–5 days. This new system may not only provide safer keratinocyte use in the clinics but also facilitate the broader use of other cultured human epithelial cells in regenerative medicine.
The cell-matrix interactions play a critical role in keratinocyte cellular behavior, wound repair and regeneration
The epidermal BM also contains laminin 332 which has been shown to support stable anchoring of basal keratinocytes to the epidermal basement membrane and to function as a cell motility factor during wound healing. Mutations in the genes that encode the laminin 332 subunits abrogate or perturb the functions of laminin 332 which may cause disruption of the epithelial adhesion complex, resulting in a skin blistering disease known as epidermolysis bullosa. Laminin 332 promotes the fast-spreading of epithelial cells and functions as a motility factor for wound healing. Laminin 332 also plays a key role and cancer invasion.
Ishihara et al. (2018) showed that multiple laminin isoforms promiscuously bind to growth factors (GFs)with high affinity, through their heparin-binding domains located in the α chain laminin-type G (LG) domains. The authors explore the application of these multifunctional laminin HBDs in wound healing in the type-2 diabetic mouse and demonstrate that covalent incorporation of laminin HBDs into fibrin matrices improve retention of GFs and significantly enhances the efficacy of vascular endothelial cell growth factor (VEGF-A165) and platelet-derived growth factor (PDGF-BB) in promoting wound healing in vivo, under conditions where the GFs alone in fibrin are inefficacious.
Succeed with your application
-
Instructions 001: Coating with Biolaminin substrates
Protocol and concentration calculations for coating cultureware with BiolamininView post -
Application note 016: Biosilk 3D biomaterial for organoid culture
Features and supporting data for Biosilk in 3D cell cultureView post -
Application note 017: Xeno-free and defined keratinocyte culture on Biolaminin® 521 and Biolaminin 511 substrates
Xeno-free and defined keratinocyte culture on Biolaminin® 521 and Biolaminin 511 substratesView post -
Instructions 003: Culturing human ES and iPS cells
Protocol for the transition of human PSCs to Biolaminin cell culture matrices (LN, MX, and CTG)View post -
Chemically defined and xenogeneic-free culture method for human epidermal keratinocytes on laminin-based matrices
Chemically defined and xenogeneic-free culture method for human epidermal keratinocytes on laminin-based matrices Tjin M.S., Chua A.W.C, Tryggvason K. Nature Protocols, […]View publication -
Application note 023: Next generation xeno-free and defined skin cell culture – on Biolaminin® 521 (LN521) and Biolaminin 511 (LN511) substrates
Skin is the largest organ of the body and our first line of defense against pathogens and environmental impacts. The skin is composed of two main layers, epidermis and dermis. They are separated by an ultrathin basement membrane (BM), highly enriched in laminin proteins, particularly the isoforms 332, 331, 521 and 511. Laminin 332/331 are mostly associated with adhesion and maturation and 521/511 with adhesion and proliferation. The complex interaction of BM and cells is essential for healthy skin development and tissue homeostasis, and essential part of the stem cell niche of the skin.View post
Biolaminin Key Advantages
Laminin-511 and laminin-521 are potent adhesive substrates for both neonatal and adult keratinocytes and this adhesion is mediated by the α3β1 and α6β4 integrins. Laminin-332 promotes a rapid spreading of epithelial cells and functions as a motility factor for wound healing and cancer invasion.
Specific laminin isoforms are present in different tissue microenvironments and are essential for cell survival, proliferation, and differentiation. Biolaminin products allow you to imitate the natural cell-matrix interactions in vitro.
All our matrices are chemically defined and animal origin-free, which makes them ideal substrates for each level of the scientific process – from basic research to clinical applications.
Our products have consistent composition and quality. This enables minimized variability between experiments.
Numerous scientists have found our products and finally succeeded in their specific stem cell application. The power of full-length laminins incorporated into various cell systems is well documented in scientific articles and clinical trials.
Recommended products
-

Biolaminin 332 LN (LN332)
Full-length human recombinant laminin-332
Biolaminin 332 supports cells in epithelial basement membranes located in several tissues.View product -

Biolaminin 511 LN (LN511)
Full-length human recombinant laminin-511
Biolaminin 511 is the natural laminin for mouse embryonic stem cells, supporting sustained pluripotency without the need for LIF. It also efficiently promotes the culture of many tissue-specific human cell types.View product -

Biolaminin 521 LN (LN521)
Full-length human recombinant laminin-521
Biolaminin 521 LN is a full-length laminin-521 substrate—the natural laminin for pluripotent stem cells, reliably facilitating ESC and iPSC self-renewal in a chemically defined, xeno-free stem cell culture system. It also uniquely promotes the growth and identity of various tissue-specific human cell types.View product

Talk to our team for customized support
We are here to help you in your journey.